TLR-9 agonist and CD40-targeting vaccination induces HIV-1 envelope-specific B cells with a diversified immunoglobulin repertoire in humanized mice
- PMID: 33253297
- PMCID: PMC7728200
- DOI: 10.1371/journal.ppat.1009025
TLR-9 agonist and CD40-targeting vaccination induces HIV-1 envelope-specific B cells with a diversified immunoglobulin repertoire in humanized mice
Abstract
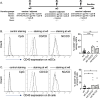
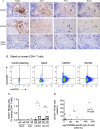
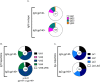
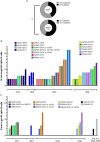
The development of HIV-1 vaccines is challenged by the lack of relevant models to accurately induce human B- and T-cell responses in lymphoid organs. In humanized mice reconstituted with human hematopoietic stem cells (hu-mice), human B cell-development and function are impaired and cells fail to efficiently transition from IgM B cells to IgG B cells. Here, we found that CD40-targeted vaccination combined with CpG-B adjuvant overcomes the usual defect of human B-cell switch and maturation in hu-mice. We further dissected hu-B cell responses directed against the HIV-1 Env protein elicited by targeting Env gp140 clade C to the CD40 receptor of antigen-presenting cells. The anti-CD40.Env gp140 vaccine was injected with CpG-B in a homologous prime/boost regimen or as a boost of a NYVAC-KC pox vector encoding Env gp140 clade C. Both regimens elicited Env-specific IgG-switched memory hu-B cells at a greater magnitude in hu-mice primed with NYVAC-KC. Single-cell RNA-seq analysis showed gp140-specific hu-B cells to express polyclonal IgG1 and IgG3 isotypes and a broad Ig VH/VL repertoire, with predominant VH3 family gene usage. These cells exhibited a higher rate of somatic hypermutation than the non-specific IgG+ hu-B-cell counterpart. Both vaccine regimens induced splenic GC-like structures containing hu-B and hu-Tfh-like cells expressing PD-1 and BCL-6. We confirmed in this model that circulating ICOS+ memory hu-Tfh cells correlated with the magnitude of gp140-specific B-cell responses. Finally, the NYVAC-KC heterologous prime led to a more diverse clonal expansion of specific hu-B cells. Thus, this study shows that CD40-targeted vaccination induces human IgG production in hu-mice and provides insights for the development of a CD40-targeting vaccine to prevent HIV-1 infection in humans.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: GZ, SZ, and YL are named inventors on CD40-targeting vaccine patents and patent filings held jointly by INSERM and the Baylor Research Institute.
Figures



References
-
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372(9653):1881–93. 10.1016/S0140-6736(08)61591-3 - DOI - PMC - PubMed
-
- Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, Nchabeleng M, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011;11(7):507–15. 10.1016/S1473-3099(11)70098-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

