Anlotinib for refractory advanced non-small-cell lung cancer: A systematic review and meta-analysis
- PMID: 33253313
- PMCID: PMC7703897
- DOI: 10.1371/journal.pone.0242982
Anlotinib for refractory advanced non-small-cell lung cancer: A systematic review and meta-analysis
Abstract
Objective: To assess the efficacy and toxicity of anlotinib for the treatment of refractory advanced non-small-cell lung cancer (NSCLC).
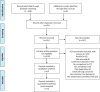
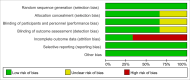
Methods: We systematically searched databases for randomized controlled trials on anlotinib treatment for patients with advanced NSCLC published until November 6, 2020. Articles were assessed and data were extracted independently by two investigators. Further, we analyzed hazard ratios (HRs) for progression-free and overall survival (PFS and OS, respectively). In addition, we analyzed risk ratio (RR) for overall response and disease control rates (ORR and DCR, respectively) and the odds ratio (OR) for the main adverse events (AEs) using RevMan 5.3 software.
Results: This analysis included 594 patients from three clinical studies. The pooled HRs for PFS and OS were 0.27 (95% confidence interval (CI): 0.22-0.33, P < 0.001) and 0.68 (95% CI: 0.56-0.83, P < 0.001), respectively, indicating that anlotinib administration significantly improved PFS and OS in patients with advanced NSCLC. The pooled RRs for ORR and DCR were 11.62 (95% CI: 2.75-49.14, P < 0.001) and 2.30 (95% CI: 1.91-2.77, P < 0.001), respectively, indicating that anlotinib administration in patients with advanced NSCLC improved ORR and DCR. The pooled OR for AEs of grade 3 or higher was 2.94 (95% CI: 1.99-4.35, P < 0.001), indicating that AEs of grade 3 or higher were more prevalent in the anlotinib group than in the placebo group.
Conclusion: Anlotinib, an effective choice of third- or later line therapy for patients with refractory advanced NSCLC, provides clinical benefits in terms of PFS, OS, ORR, and DCR. AEs associated with anlotinib were tolerable.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
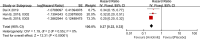
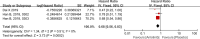

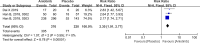
Similar articles
-
Response to first-line treatment predicts progression-free survival benefit of small-cell lung cancer patients treated with anlotinib.Cancer Med. 2021 Jun;10(12):3896-3904. doi: 10.1002/cam4.3941. Epub 2021 May 6. Cancer Med. 2021. PMID: 33960145 Free PMC article.
-
China National Medical Products Administration approval summary: anlotinib for the treatment of advanced non-small cell lung cancer after two lines of chemotherapy.Cancer Commun (Lond). 2019 Jun 20;39(1):36. doi: 10.1186/s40880-019-0383-7. Cancer Commun (Lond). 2019. PMID: 31221221 Free PMC article. Review.
-
Efficacy and safety of anlotinib monotherapy or combination therapy in the treatment of patients with advanced non-small cell lung cancer: a retrospective real-world study conducted in East China.BMC Pulm Med. 2025 Apr 10;25(1):170. doi: 10.1186/s12890-025-03635-8. BMC Pulm Med. 2025. PMID: 40211232 Free PMC article.
-
Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302).Br J Cancer. 2018 Mar 6;118(5):654-661. doi: 10.1038/bjc.2017.478. Epub 2018 Feb 13. Br J Cancer. 2018. PMID: 29438373 Free PMC article. Clinical Trial.
-
Anlotinib, a novel TKI, as a third-line or further-line treatment in patients with advanced non-small cell lung cancer in China: A systemic review and meta-analysis of its efficacy and safety.Medicine (Baltimore). 2021 Jun 11;100(23):e25709. doi: 10.1097/MD.0000000000025709. Medicine (Baltimore). 2021. PMID: 34114981 Free PMC article.
Cited by
-
Traditional Chinese Medicine Brucea Javanica Oil Enhances the Efficacy of Anlotinib in a Mouse Model of Liver-Metastasis of Small-cell Lung Cancer.In Vivo. 2021 May-Jun;35(3):1437-1441. doi: 10.21873/invivo.12395. In Vivo. 2021. PMID: 33910820 Free PMC article.
-
Efficacy and safety of anlotinib as a third-line treatment of advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials.Oncol Lett. 2022 May 27;24(1):229. doi: 10.3892/ol.2022.13350. eCollection 2022 Jul. Oncol Lett. 2022. PMID: 35720500 Free PMC article.
-
Role of drug-eluting bead bronchial arterial chemoembolisation in the treatment of non-small cell lung cancer: protocol for a meta-analysis.BMJ Open. 2024 Jul 1;14(6):e079038. doi: 10.1136/bmjopen-2023-079038. BMJ Open. 2024. PMID: 38951003 Free PMC article.
-
Anlotinib-containing regimen for advanced small-cell lung cancer: A protocol of meta-analysis.PLoS One. 2021 Mar 11;16(3):e0247494. doi: 10.1371/journal.pone.0247494. eCollection 2021. PLoS One. 2021. PMID: 33705427 Free PMC article.
-
The Value of Anlotinib in the Treatment of Intractable Brain Edema: Two Case Reports.Front Oncol. 2021 Mar 22;11:617803. doi: 10.3389/fonc.2021.617803. eCollection 2021. Front Oncol. 2021. PMID: 33828975 Free PMC article.
References
-
- Li N, Ou W, Ye X, Sun HB, Zhang L, Fang Q, et al. Pemetrexed-carboplatin adjuvant chemotherapy with or without gefitinib in resected stage IIIA-N2 non-small cell lung cancer harbouring EGFR mutations: a randomized, phase II study. Ann Surg Oncol. 2014;21(6):2091–6. 10.1245/s10434-014-3586-9 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

