Brain cancer induces systemic immunosuppression through release of non-steroid soluble mediators
- PMID: 33253355
- PMCID: PMC7954397
- DOI: 10.1093/brain/awaa343
Brain cancer induces systemic immunosuppression through release of non-steroid soluble mediators
Abstract
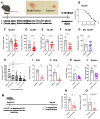
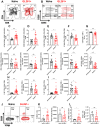
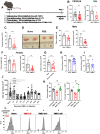
Immunosuppression of unknown aetiology is a hallmark feature of glioblastoma and is characterized by decreased CD4 T-cell counts and downregulation of major histocompatibility complex class II expression on peripheral blood monocytes in patients. This immunosuppression is a critical barrier to the successful development of immunotherapies for glioblastoma. We recapitulated the immunosuppression observed in glioblastoma patients in the C57BL/6 mouse and investigated the aetiology of low CD4 T-cell counts. We determined that thymic involution was a hallmark feature of immunosuppression in three distinct models of brain cancer, including mice harbouring GL261 glioma, B16 melanoma, and in a spontaneous model of diffuse intrinsic pontine glioma. In addition to thymic involution, we determined that tumour growth in the brain induced significant splenic involution, reductions in peripheral T cells, reduced MHC II expression on blood leucocytes, and a modest increase in bone marrow resident CD4 T cells. Using parabiosis we report that thymic involution, declines in peripheral T-cell counts, and reduced major histocompatibility complex class II expression levels were mediated through circulating blood-derived factors. Conversely, T-cell sequestration in the bone marrow was not governed through circulating factors. Serum isolated from glioma-bearing mice potently inhibited proliferation and functions of T cells both in vitro and in vivo. Interestingly, the factor responsible for immunosuppression in serum is non-steroidal and of high molecular weight. Through further analysis of neurological disease models, we determined that the immunosuppression was not unique to cancer itself, but rather occurs in response to brain injury. Non-cancerous acute neurological insults also induced significant thymic involution and rendered serum immunosuppressive. Both thymic involution and serum-derived immunosuppression were reversible upon clearance of brain insults. These findings demonstrate that brain cancers cause multifaceted immunosuppression and pinpoint circulating factors as a target of intervention to restore immunity.
Keywords: T cells; glioblastoma; immunosuppression; neuroimmunology; thymus; .
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures





References
-
- Antonica A, Magni F, Mearini L, Paolocci N.. Vegal control of lymphocyte release from rat thymus. J Auton Nervous Syst 1994; 48: 187–97. - PubMed
-
- Ayasoufi K, Pfaller CK, Khadka RH, Jin F, Johnson AJ.. Brain-Thymus communication is a novel immunosuppressive feature of neurological insults. J Immunol 2019; 202(1 Supplement):183.19.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

