Proteome-Scale Analysis of Protein S-Acylation Comes of Age
- PMID: 33253586
- PMCID: PMC7775881
- DOI: 10.1021/acs.jproteome.0c00409
Proteome-Scale Analysis of Protein S-Acylation Comes of Age
Abstract
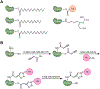
Protein S-acylation (commonly known as palmitoylation) is a widespread reversible lipid modification, which plays critical roles in regulating protein localization, activity, stability, and complex formation. The deregulation of protein S-acylation contributes to many diseases such as cancer and neurodegenerative disorders. The past decade has witnessed substantial progress in proteomic analysis of protein S-acylation, which significantly advanced our understanding of S-acylation biology. In this review, we summarized the techniques for the enrichment of S-acylated proteins or peptides, critically reviewed proteomic studies of protein S-acylation at eight different levels, and proposed major challenges for the S-acylproteomics field. In summary, proteome-scale analysis of protein S-acylation comes of age and will play increasingly important roles in discovering new disease mechanisms, biomarkers, and therapeutic targets.
Keywords: ABE; APT; MLCC; PAT; S-acylation; S-palmitoylation; click chemistry; palmitoylation; proteomics.
Figures

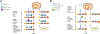

References
-
- Linder ME; Deschenes RJ Palmitoylation: Policing Protein Stability and Traffic. Nat. Rev. Mol. Cell Biol 2007, 8 (1), 74–84. - PubMed
-
- Muszbek L; Laposata M Covalent Modification of Platelet Proteins by Palmitate. Blood 1989, 74 (4), 1339–1347. - PubMed
-
- Muszbek L; Laposata M Myristoylation of Proteins in Platelets Occurs Predominantly through Thioester Linkages. J. Biol. Chem 1993, 268 (11), 8251–8255. - PubMed
-
- Muszbek L; Laposata M Covalent Modification of Proteins by Arachidonate and Eicosapentaenoate in Platelets. J. Biol. Chem 1993, 268 (24), 18243–18248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

