c-Jun N-terminal Kinase Mediates Ligand-independent p75NTR Signaling in Mesencephalic Cells Subjected to Oxidative Stress
- PMID: 33253821
- PMCID: PMC8783671
- DOI: 10.1016/j.neuroscience.2020.11.036
c-Jun N-terminal Kinase Mediates Ligand-independent p75NTR Signaling in Mesencephalic Cells Subjected to Oxidative Stress
Abstract
The p75 neurotrophin receptor (p75NTR) is a multifunctional protein that regulates cellular responses to pathological conditions in specific regions of the nervous system. Activation of p75NTR in certain neuronal populations induces proteolytic processing of the receptor, thereby generating p75NTR fragments that facilitate downstream signaling. Expression of p75NTR has been reported in neurons of the ventral midbrain, but p75NTR signaling mechanisms in such cells are poorly understood. Here, we used Lund Human Mesencephalic cells, a population of neuronal cells derived from the ventral mesencephalon, to evaluate the effects of oxidative stress on p75NTR signaling. Subjection of the cells to oxidative stress resulted in decreased cell-surface localization of p75NTR and intracellular accumulation of p75NTR fragments. Oxidative stress-induced p75NTR processing was reduced by pharmacological inhibition of metalloproteases or γ-secretase, but was unaltered by blockade of the ligand-binding domain of p75NTR. Furthermore, inhibition of c-Jun N-terminal Kinase (JNK) decreased p75NTR cleavage induced by oxidative damage. Altogether, these results support a mechanism of p75NTR activation in which oxidative stress stimulates JNK signaling, thereby facilitating p75NTR processing via a ligand-independent mechanism involving induction of metalloprotease and γ-secretase activity. These findings reveal a novel role for JNK in ligand-independent p75NTR signaling, and, considering the susceptibility of mesencephalic neurons to oxidative damage associated with Parkinson's disease (PD), merit further investigation into the effects of p75NTR on PD-related neurodegeneration.
Keywords: JNK; Parkinson’s disease; neurotrophin; p75(NTR); proteolysis; reactive oxygen species.
Copyright © 2020 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures


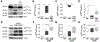



References
-
- Angelova PR and Abramov AY (2018) Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett 592, 692–702. - PubMed
-
- Auladell C, de Lemos L, Verdaguer E et al. (2017) Role of JNK isoforms in the kainic acid experimental model of epilepsy and neurodegeneration. Front Biosci (Landmark Ed) 22, 795–814. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

