Biophysical processes underlying cross-seeding in amyloid aggregation and implications in amyloid pathology
- PMID: 33254009
- PMCID: PMC10317075
- DOI: 10.1016/j.bpc.2020.106507
Biophysical processes underlying cross-seeding in amyloid aggregation and implications in amyloid pathology
Abstract
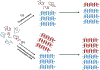
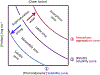
Abnormal aggregation of proteins into filamentous aggregates commonly associates with many diseases, such as Alzheimer's disease, Parkinson's disease and type-2 diabetes. These filamentous aggregates, also known as amyloids, can propagate their abnormal structures to either the same precursor molecules (seeding) or other protein monomers (cross-seeding). Cross-seeding has been implicated in the abnormal protein aggregation and has been found to facilitate the formation of physiological amyloids. It has risen to be an exciting area of research with a high volume of published reports. In this review article, we focus on the biophysical processes underlying the cross-seeding for some of the most commonly studied amyloid proteins. Here we will discuss the relevant literature related to cross-seeded polymerization of amyloid-beta, human islet amyloid polypeptide (hIAPP, or also known as amylin) and alpha-synuclein. SEVI (semen-derived enhancer of viral infection) amyloid formation by the cross-seeding between the bacterial curli protein and PAP248-286 is also briefly discussed.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest
None.
Figures







Similar articles
-
Fluorescence-Based Monitoring of Early-Stage Aggregation of Amyloid-β, Amylin Peptide, Tau, and α-Synuclein Proteins.ACS Chem Neurosci. 2024 Sep 4;15(17):3113-3123. doi: 10.1021/acschemneuro.4c00097. Epub 2024 Aug 16. ACS Chem Neurosci. 2024. PMID: 39150403 Free PMC article.
-
Studies on the cross-interaction between hIAPP and Aβ25-35 and the aggregation process in binary mixture by electrospray ionization-ion mobility-mass spectrometry.J Mass Spectrom. 2020 Oct;55(10):e4643. doi: 10.1002/jms.4643. J Mass Spectrom. 2020. PMID: 32893436
-
Islet amyloid polypeptide fibril catalyzes amyloid-β aggregation by promoting fibril nucleation rather than direct axial growth.Int J Biol Macromol. 2024 Nov;279(Pt 1):135137. doi: 10.1016/j.ijbiomac.2024.135137. Epub 2024 Aug 27. Int J Biol Macromol. 2024. PMID: 39208885
-
The amyloid state of proteins: A boon or bane?Int J Biol Macromol. 2022 Mar 1;200:593-617. doi: 10.1016/j.ijbiomac.2022.01.115. Epub 2022 Jan 22. Int J Biol Macromol. 2022. PMID: 35074333 Review.
-
Characterisation of the Structure and Oligomerisation of Islet Amyloid Polypeptides (IAPP): A Review of Molecular Dynamics Simulation Studies.Molecules. 2018 Aug 25;23(9):2142. doi: 10.3390/molecules23092142. Molecules. 2018. PMID: 30149632 Free PMC article. Review.
Cited by
-
Co-aggregation and secondary nucleation in the life cycle of human prolactin/galanin functional amyloids.Elife. 2022 Mar 8;11:e73835. doi: 10.7554/eLife.73835. Elife. 2022. PMID: 35257659 Free PMC article.
-
Amyloid-β peptide 37, 38 and 40 individually and cooperatively inhibit amyloid-β 42 aggregation.Chem Sci. 2022 Feb 7;13(8):2423-2439. doi: 10.1039/d1sc02990h. eCollection 2022 Feb 23. Chem Sci. 2022. PMID: 35310497 Free PMC article.
-
Direct observation of protein structural transitions through entire amyloid aggregation processes in water using 2D-IR spectroscopy.Chem Sci. 2022 Mar 18;13(16):4482-4489. doi: 10.1039/d1sc06047c. eCollection 2022 Apr 20. Chem Sci. 2022. PMID: 35656138 Free PMC article.
-
Dynamic protein structures in normal function and pathologic misfolding in systemic amyloidosis.Biophys Chem. 2022 Jan;280:106699. doi: 10.1016/j.bpc.2021.106699. Epub 2021 Oct 14. Biophys Chem. 2022. PMID: 34773861 Free PMC article. Review.
-
Expression of N-Terminal Cysteine Aβ42 and Conjugation to Generate Fluorescent and Biotinylated Aβ42.Biochemistry. 2021 Apr 20;60(15):1191-1200. doi: 10.1021/acs.biochem.1c00105. Epub 2021 Apr 1. Biochemistry. 2021. PMID: 33793198 Free PMC article.
References
-
- Chiti F, Dobson CM, Protein misfolding, amyloid formation, and human disease: a summary of progress over the last decade, Annu. Rev. Biochem 86 (2017) 27–68. - PubMed
-
- Sunde M, Serpell LC, Bartlam M, Fraser PE, Pepys MB, Blake CC, Common core structure of amyloid fibrils by synchrotron X-ray diffraction, J. Mol. Biol 273 (1997) 729–739. - PubMed
-
- Iadanza MG, Jackson MP, Hewitt EW, Ranson NA, Radford SE, A new era for understanding amyloid structures and disease, Nat. Rev. Mol. Cell Biol 19 (2018) 755–773. - PubMed
-
- Jarrett JT, Lansbury PT Jr., Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell 73 (1993) 1055–1058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

