Modulation of OSCP mitigates mitochondrial and synaptic deficits in a mouse model of Alzheimer's pathology
- PMID: 33254080
- PMCID: PMC7923248
- DOI: 10.1016/j.neurobiolaging.2020.09.018
Modulation of OSCP mitigates mitochondrial and synaptic deficits in a mouse model of Alzheimer's pathology
Abstract
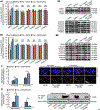
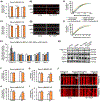
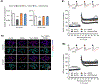
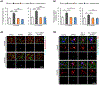
Synaptic failure underlies cognitive impairment in Alzheimer's disease (AD). Cumulative evidence suggests a strong link between mitochondrial dysfunction and synaptic deficits in AD. We previously found that oligomycin-sensitivity-conferring protein (OSCP) dysfunction produces pronounced neuronal mitochondrial defects in AD brains and a mouse model of AD pathology (5xFAD mice). Here, we prevented OSCP dysfunction by overexpressing OSCP in 5xFAD mouse neurons in vivo (Thy-1 OSCP/5xFAD mice). This approach protected OSCP expression and reduced interaction of amyloid-beta (Aβ) with membrane-bound OSCP. OSCP overexpression also alleviated F1Fo ATP synthase deregulation and preserved mitochondrial function. Moreover, OSCP modulation conferred resistance to Aβ-mediated defects in axonal mitochondrial dynamics and motility. Consistent with preserved neuronal mitochondrial function, OSCP overexpression ameliorated synaptic injury in 5xFAD mice as demonstrated by preserved synaptic density, reduced complement-dependent synapse elimination, and improved synaptic transmission, leading to preserved spatial learning and memory. Taken together, our findings show the consequences of OSCP dysfunction in the development of synaptic stress in AD-related conditions and implicate OSCP modulation as a potential therapeutic strategy.
Keywords: Alzheimer's disease; Amyloid beta; Mitochondrial F1Fo ATP synthase; Oligomycin-sensitivity-conferring protein; Synaptic injury.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
COMPETING INTERESTS
The authors have no conflict of interest to claim.
Figures

Similar articles
-
Cyclophilin D deficiency attenuates mitochondrial F1Fo ATP synthase dysfunction via OSCP in Alzheimer's disease.Neurobiol Dis. 2019 Jan;121:138-147. doi: 10.1016/j.nbd.2018.09.020. Epub 2018 Sep 26. Neurobiol Dis. 2019. PMID: 30266287 Free PMC article.
-
Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer's disease.Nat Commun. 2016 May 6;7:11483. doi: 10.1038/ncomms11483. Nat Commun. 2016. PMID: 27151236 Free PMC article.
-
Cyclophilin D Promotes Brain Mitochondrial F1FO ATP Synthase Dysfunction in Aging Mice.J Alzheimers Dis. 2017;55(4):1351-1362. doi: 10.3233/JAD-160822. J Alzheimers Dis. 2017. PMID: 27834780 Free PMC article.
-
Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer's Disease.J Alzheimers Dis. 2021;84(4):1391-1414. doi: 10.3233/JAD-215139. J Alzheimers Dis. 2021. PMID: 34719499 Review.
-
Unveiling OSCP as the potential therapeutic target for mitochondrial dysfunction-related diseases.Life Sci. 2024 Jan 1;336:122293. doi: 10.1016/j.lfs.2023.122293. Epub 2023 Nov 27. Life Sci. 2024. PMID: 38030056 Review.
Cited by
-
Mitochondrial Dysfunction Links to Impaired Hippocampal Serotonin Release in a Mouse Model of Alzheimer's Disease.J Alzheimers Dis. 2023;93(2):605-619. doi: 10.3233/JAD-230072. J Alzheimers Dis. 2023. PMID: 37066917 Free PMC article.
-
Contrasting Effects of an Atherogenic Diet and High-Protein/Unsaturated Fatty Acids Diet on the Accelerated Aging Mouse Model SAMP8 Phenotype.Neurol Int. 2024 Sep 23;16(5):1066-1085. doi: 10.3390/neurolint16050080. Neurol Int. 2024. PMID: 39452682 Free PMC article.
-
Latest assessment methods for mitochondrial homeostasis in cognitive diseases.Neural Regen Res. 2024 Apr;19(4):754-768. doi: 10.4103/1673-5374.382222. Neural Regen Res. 2024. PMID: 37843209 Free PMC article. Review.
-
Mitochondrial Permeability Transition: A Pore Intertwines Brain Aging and Alzheimer's Disease.Cells. 2021 Mar 15;10(3):649. doi: 10.3390/cells10030649. Cells. 2021. PMID: 33804048 Free PMC article. Review.
-
The use of fibroblasts as a valuable strategy for studying mitochondrial impairment in neurological disorders.Transl Neurodegener. 2022 Jul 4;11(1):36. doi: 10.1186/s40035-022-00308-y. Transl Neurodegener. 2022. PMID: 35787292 Free PMC article. Review.
References
-
- Adelstein TB, Kesner RP, Strassberg DS, 1992. Spatial recognition and spatial order memory in patients with dementia of the Alzheimer’s type. Neuropsychologia 30(1), 59–67. - PubMed
-
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr., Jonas EA, 2014. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111(29), 10580–10585. - PMC - PubMed
-
- Bonora M, Morganti C, Morciano G, Pedriali G, Lebiedzinska-Arciszewska M, Aquila G, Giorgi C, Rizzo P, Campo G, Ferrari R, Kroemer G, Wieckowski MR, Galluzzi L, Pinton P, 2017. Mitochondrial permeability transition involves dissociation of F1FO ATP synthase dimers and C-ring conformation. EMBO Rep 18(7), 1077–1089. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

