Premises among SARS-CoV-2, dysbiosis and diarrhea: Walking through the ACE2/mTOR/autophagy route
- PMID: 33254549
- PMCID: PMC7467124
- DOI: 10.1016/j.mehy.2020.110243
Premises among SARS-CoV-2, dysbiosis and diarrhea: Walking through the ACE2/mTOR/autophagy route
Abstract
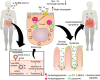
Recently, a new coronavirus (SARS-CoV-2) was discovered in China. Due to its high level of contagion, it has already reached most countries, quickly becoming a pandemic. Although the most common symptoms are related to breathing problems, SARS-CoV-2 infections also affect the gastrointestinal tract culminating in inflammation and diarrhea. However, the mechanisms related to these enteric manifestations are still not well understood. Evidence shows that the SARS-CoV-2 binds to the angiotensin-converting enzyme receptor 2 (ACE2) in host cells as a viral invasion mechanism and can infect the lungs and the gut. Other viruses have already been linked to intestinal symptoms through binding to ACE2. In turn, this medical hypothesis article conjectures that the ACE2 downregulation caused by the SARS-CoV-2 internalization could lead to decreased activation of the mechanistic target of mTOR with increased autophagy and lead to intestinal dysbiosis, resulting in diarrhea. Besides that, dysbiosis can directly affect the respiratory system through the lungs. Although there are clues to other viruses that modulate the ACE2/gut/lungs axis, including the participation of autophagy and dysbiosis in the development of gastrointestinal symptoms, there is still no evidence of the ACE2/mTOR/autophagy pathway in SARS-CoV-2 infections. Thus, we propose that the new coronavirus causes a change in the intestinal microbiota, which culminates in a diarrheal process through the ACE2/mTOR/autophagy pathway into enterocytes. Our assumption is supported by premises that unregulated intestinal microbiota increases the susceptibility to other diseases and extra-intestinal manifestations, which can even cause remote damage in lungs. These putative connections lead us to suggest and encourage future studies aiming at assessing the aforementioned hypothesis and regulating dysbiosis caused by SARS-CoV-2 infection, in order to confirm the decrease in lung injuries and the improvement in the prognosis of the disease.
Keywords: Coronavirus; Diarrhea; Dysbiosis; SARS-CoV-2.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities - Role of gut microbiota dysbiosis.Ageing Res Rev. 2020 Sep;62:101123. doi: 10.1016/j.arr.2020.101123. Epub 2020 Jul 16. Ageing Res Rev. 2020. PMID: 32683039 Free PMC article. Review.
-
Immunological co-ordination between gut and lungs in SARS-CoV-2 infection.Virus Res. 2020 Sep;286:198103. doi: 10.1016/j.virusres.2020.198103. Epub 2020 Jul 24. Virus Res. 2020. PMID: 32717345 Free PMC article. Review.
-
Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2.Circ Res. 2020 May 8;126(10):1456-1474. doi: 10.1161/CIRCRESAHA.120.317015. Epub 2020 Apr 8. Circ Res. 2020. PMID: 32264791 Free PMC article. Review.
-
Relevance of SARS-CoV-2 related factors ACE2 and TMPRSS2 expressions in gastrointestinal tissue with pathogenesis of digestive symptoms, diabetes-associated mortality, and disease recurrence in COVID-19 patients.Med Hypotheses. 2020 Nov;144:110271. doi: 10.1016/j.mehy.2020.110271. Epub 2020 Sep 13. Med Hypotheses. 2020. PMID: 33254575 Free PMC article.
-
Angiotensin-converting enzyme 2 and COVID-19 in cardiorenal diseases.Clin Sci (Lond). 2021 Jan 15;135(1):1-17. doi: 10.1042/CS20200482. Clin Sci (Lond). 2021. PMID: 33399851 Free PMC article. Review.
Cited by
-
The Concept of Intrauterine Programming and the Development of the Neonatal Microbiome in the Prevention of SARS-CoV-2 Infection.Nutrients. 2022 Apr 20;14(9):1702. doi: 10.3390/nu14091702. Nutrients. 2022. PMID: 35565670 Free PMC article. Review.
-
The relationship between gut microbiota and COVID-19 progression: new insights into immunopathogenesis and treatment.Front Immunol. 2023 May 2;14:1180336. doi: 10.3389/fimmu.2023.1180336. eCollection 2023. Front Immunol. 2023. PMID: 37205106 Free PMC article. Review.
-
Symptom clusters and symptom networks of symptom experiences in patients with SARS-CoV-2 infection.Heliyon. 2024 Nov 19;10(22):e40497. doi: 10.1016/j.heliyon.2024.e40497. eCollection 2024 Nov 30. Heliyon. 2024. PMID: 39650177 Free PMC article.
-
Direct, indirect, post-infection damages induced by coronavirus in the human body: an overview.Virusdisease. 2022 Dec;33(4):429-444. doi: 10.1007/s13337-022-00793-9. Epub 2022 Oct 25. Virusdisease. 2022. PMID: 36311173 Free PMC article. Review.
-
Alterations of the gut microbiota in coronavirus disease 2019 and its therapeutic potential.World J Gastroenterol. 2022 Dec 21;28(47):6689-6701. doi: 10.3748/wjg.v28.i47.6689. World J Gastroenterol. 2022. PMID: 36620345 Free PMC article. Review.
References
-
- Masters P.S., Perlman S. Coronaviridae. Fields Virology. 2013;6(1):825–858.
-
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). https://www.who.int/emergencies/mers-cov/en/ (accessed April 14, 2020).
-
- World Health Organization. Cumulative number of reported probable cases of Severe Acute Respiratory Syndrome (SARS) https://www.who.int/csr/sars/country/en/ (accessed April 14, 2020).
-
- Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N.W., Ke R. The novel coronavirus, 2019-nCoV, is highly contagious and more infectious than initially estimated. ArXiv.org. 2020:1–34. https://arxiv.org/abs/2002.03268.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

