Evaluation of remodeling and regeneration of electrospun PCL/fibrin vascular grafts in vivo
- PMID: 33255034
- PMCID: PMC7445127
- DOI: 10.1016/j.msec.2020.111441
Evaluation of remodeling and regeneration of electrospun PCL/fibrin vascular grafts in vivo
Abstract
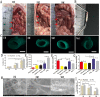
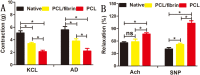
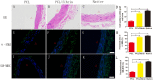
The success of artificial vascular graft in the host to obtain functional tissue regeneration and remodeling is a great challenge in the field of small diameter tissue engineering blood vessels. In our previous work, poly(ε-caprolactone) (PCL)/fibrin vascular grafts were fabricated by electrospinning. It was proved that the PCL/fibrin vascular graft was a suitable small diameter tissue engineering vascular scaffold with good biomechanical properties and cell compatibility. Here we mainly examined the performance of PCL/fibrin vascular graft in vivo. The graft showed randomly arranged nanofiber structure, excellent mechanical strength, higher compliance and degradation properties. At 9 months after implantation in the rat abdominal aorta, the graft induced the regeneration of neoarteries, and promoted ECM deposition and rapid endothelialization. More importantly, the PCL/fibrin vascular graft showed more microvessels density and fewer calcification areas at 3 months, which was beneficial to improve cell infiltration and proliferation. Moreover, the ratio of M2/M1macrophage in PCL/fibrin graft had a higher expression level and the secretion amount of pro-inflammatory cytokines started to increase, and then decreased to similar to the native artery. Thus, the electrospun PCL/fibrin tubular vascular graft had great potential to become a new type of artificial blood vessel scaffold that can be implanted in vivo for long term.
Keywords: Electrospinning; In vivo; Inflammatory cytokines; PCL/fibrin vascular grafts; Tissue remodeling and regeneration.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Circumferentially Aligned Electrospun Vascular Grafts Improves Its Vascular Regeneration and Remodeling in vivo.Int J Nanomedicine. 2025 Jun 14;20:7515-7532. doi: 10.2147/IJN.S518283. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40535837 Free PMC article.
-
The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration.Biomaterials. 2014 Jul;35(22):5700-10. doi: 10.1016/j.biomaterials.2014.03.078. Epub 2014 Apr 17. Biomaterials. 2014. PMID: 24746961
-
Development of a decellularized human amniotic membrane-based electrospun vascular graft capable of rapid remodeling for small-diameter vascular applications.Acta Biomater. 2022 Oct 15;152:144-156. doi: 10.1016/j.actbio.2022.09.009. Epub 2022 Sep 13. Acta Biomater. 2022. PMID: 36108966
-
Electrospun nanofiber scaffold for vascular tissue engineering.Mater Sci Eng C Mater Biol Appl. 2021 Oct;129:112373. doi: 10.1016/j.msec.2021.112373. Epub 2021 Aug 14. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 34579892 Free PMC article. Review.
-
Polyglycerol sebacate-based elastomeric materials for arterial regeneration.J Biomed Mater Res A. 2024 Apr;112(4):574-585. doi: 10.1002/jbm.a.37583. Epub 2023 Jun 22. J Biomed Mater Res A. 2024. PMID: 37345954 Review.
Cited by
-
Challenges and strategies for in situ endothelialization and long-term lumen patency of vascular grafts.Bioact Mater. 2020 Dec 5;6(6):1791-1809. doi: 10.1016/j.bioactmat.2020.11.028. eCollection 2021 Jun. Bioact Mater. 2020. PMID: 33336112 Free PMC article. Review.
-
Controlled and Synchronised Vascular Regeneration upon the Implantation of Iloprost- and Cationic Amphiphilic Drugs-Conjugated Tissue-Engineered Vascular Grafts into the Ovine Carotid Artery: A Proteomics-Empowered Study.Polymers (Basel). 2022 Nov 26;14(23):5149. doi: 10.3390/polym14235149. Polymers (Basel). 2022. PMID: 36501545 Free PMC article.
-
Biological Materials for Tissue-Engineered Vascular Grafts: Overview of Recent Advancements.Biomolecules. 2023 Sep 14;13(9):1389. doi: 10.3390/biom13091389. Biomolecules. 2023. PMID: 37759789 Free PMC article. Review.
-
Development of a bioactive, piezoelectric PVDF-TrFE scaffold with evaluation of tissue reaction for potential in nerve repair.Biomater Sci. 2025 Sep 2. doi: 10.1039/d5bm01054c. Online ahead of print. Biomater Sci. 2025. PMID: 40892495 Free PMC article.
-
Epoxy- versus Glutaraldehyde-Treated Bovine Jugular Vein Conduit for Pulmonary Valve Replacement: A Comparison of Morphological Changes in a Pig Model.Biomedicines. 2023 Nov 20;11(11):3101. doi: 10.3390/biomedicines11113101. Biomedicines. 2023. PMID: 38002101 Free PMC article.
References
-
- Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., Chiuve S.E., Cushman M., Delling F.N., Deo R., de Ferranti S.D., Ferguson J.F., Fornage M., Gillespie C., Isasi C.R., Jimenez M.C., Jordan L.C., Judd S.E., Lackland D., Lichtman J.H., Lisabeth L., Liu S., Longenecker C.T., Lutsey P.L., Mackey J.S., Matchar D.B., Matsushita K., Mussolino M.E., Nasir K., O'Flaherty M., Palaniappan L.P., Pandey A., Pandey D.K., Reeves M.J., Ritchey M.D., Rodriguez C.J., Roth G.A., Rosamond W.D., Sampson U.K.A., Satou G.M., Shah S.H., Spartano N.L., Tirschwell D.L., Tsao C.W., Voeks J.H., Willey J.Z., Wilkins J.T., Wu J.H., Alger H.M., Wong S.S., Muntner P. Heart disease and stroke statistics-2018 Update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. - PubMed
-
- Jaspan V.N., Hines G.L. The current status of tissue-engineered vascular grafts. Cardiol. Rev. 2015;23(5):236–239. - PubMed
-
- Kannan R.Y., Salacinski H.J., Butler P.E., Hamilton G., Seifalian A.M. Current status of prosthetic bypass grafts: a review. J Biomed Mater Res B Appl Biomater. 2005;74(1):570–581. - PubMed
-
- Sologashvili T., Saat S.A., Tille J.C., De Valence S., Mugnai D., Giliberto J.P., Dillon J., Yakub A., Dimon Z., Gurny R., Walpoth B.H., Moeller M. Effect of implantation site on outcome of tissue-engineered vascular grafts. Eur. J. Pharm. Biopharm. 2019;139:272–278. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

