TRPV1 Channel: A Noxious Signal Transducer That Affects Mitochondrial Function
- PMID: 33255148
- PMCID: PMC7734572
- DOI: 10.3390/ijms21238882
TRPV1 Channel: A Noxious Signal Transducer That Affects Mitochondrial Function
Abstract
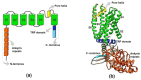
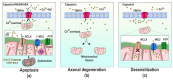
The Transient Receptor Vanilloid 1 (TRPV1) or capsaicin receptor is a nonselective cation channel, which is abundantly expressed in nociceptors. This channel is an important transducer of several noxious stimuli, having a pivotal role in pain development. Several TRPV1 studies have focused on understanding its structure and function, as well as on the identification of compounds that regulate its activity. The intracellular roles of these channels have also been explored, highlighting TRPV1's actions in the homeostasis of Ca2+ in organelles such as the mitochondria. These studies have evidenced how the activation of TRPV1 affects mitochondrial functions and how this organelle can regulate TRPV1-mediated nociception. The close relationship between this channel and mitochondria has been determined in neuronal and non-neuronal cells, demonstrating that TRPV1 activation strongly impacts on cell physiology. This review focuses on describing experimental evidence showing that TRPV1 influences mitochondrial function.
Keywords: ROS; TRPV1; apoptosis; mitochondrial dysfunction; nociception; pain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Ca2+ and calpain mediate capsaicin-induced ablation of axonal terminals expressing transient receptor potential vanilloid 1.J Biol Chem. 2017 May 19;292(20):8291-8303. doi: 10.1074/jbc.M117.778290. Epub 2017 Mar 30. J Biol Chem. 2017. PMID: 28360106 Free PMC article.
-
Privileged crosstalk between TRPV1 channels and mitochondrial calcium shuttling machinery controls nociception.Biochim Biophys Acta. 2016 Dec;1863(12):2868-2880. doi: 10.1016/j.bbamcr.2016.09.009. Epub 2016 Sep 11. Biochim Biophys Acta. 2016. PMID: 27627464
-
Activation of mitochondrial transient receptor potential vanilloid 1 channel contributes to microglial migration.Glia. 2015 Oct;63(10):1870-82. doi: 10.1002/glia.22854. Epub 2015 May 23. Glia. 2015. PMID: 26010461
-
The TRPV1 receptor and nociception.Semin Cell Dev Biol. 2006 Oct;17(5):582-91. doi: 10.1016/j.semcdb.2006.09.004. Epub 2006 Sep 24. Semin Cell Dev Biol. 2006. PMID: 17196854 Review.
-
The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1).J Physiol. 2013 Jul 1;591(13):3109-21. doi: 10.1113/jphysiol.2013.251751. Epub 2013 Apr 22. J Physiol. 2013. PMID: 23613529 Free PMC article. Review.
Cited by
-
Revolutionizing neural regeneration with smart responsive materials: Current insights and future prospects.Bioact Mater. 2025 Jun 13;52:393-421. doi: 10.1016/j.bioactmat.2025.06.003. eCollection 2025 Oct. Bioact Mater. 2025. PMID: 40585385 Free PMC article. Review.
-
Resiniferatoxin: Nature's Precision Medicine to Silence TRPV1-Positive Afferents.Int J Mol Sci. 2023 Oct 10;24(20):15042. doi: 10.3390/ijms242015042. Int J Mol Sci. 2023. PMID: 37894723 Free PMC article. Review.
-
Cytotoxic Effects of Cannabidiol on Neonatal Rat Cortical Neurons and Astrocytes: Potential Danger to Brain Development.Toxins (Basel). 2022 Oct 21;14(10):720. doi: 10.3390/toxins14100720. Toxins (Basel). 2022. PMID: 36287988 Free PMC article.
-
Complement Component C5a and Fungal Pathogen Induce Diverse Responses through Crosstalk between Transient Receptor Potential Channel (TRPs) Subtypes in Human Conjunctival Epithelial Cells.Cells. 2024 Aug 9;13(16):1329. doi: 10.3390/cells13161329. Cells. 2024. PMID: 39195219 Free PMC article.
-
TRPV1 channel antagonist capsazepine alleviates morphine tolerance and morphine-induced neurotoxicity by preventing mitochondrial damage and apoptosis: an in vivo and in vitro study.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun 23. doi: 10.1007/s00210-025-04384-5. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40549151
References
-
- Cosens D. Blindness in a Drosophila mutant. J. Insect Physiol. 1971;17:285–302. doi: 10.1016/0022-1910(71)90213-7. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

