Smart Nanofibers with Natural Extracts Prevent Senescence Patterning in a Dynamic Cell Culture Model of Human Skin
- PMID: 33255167
- PMCID: PMC7760051
- DOI: 10.3390/cells9122530
Smart Nanofibers with Natural Extracts Prevent Senescence Patterning in a Dynamic Cell Culture Model of Human Skin
Erratum in
-
Correction: Bellu et al. Smart Nanofibers with Natural Extracts Prevent Senescence Patterning in a Dynamic Cell Culture Model of Human Skin. Cells 2020, 9, 2530.Cells. 2024 Jul 31;13(15):1285. doi: 10.3390/cells13151285. Cells. 2024. PMID: 39120338 Free PMC article.
Abstract
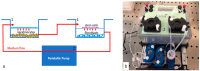
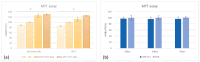
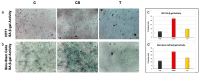
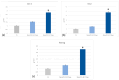
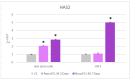
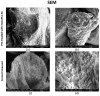
Natural cosmetic products have recently re-emerged as a novel tool able to counteract skin aging and skin related damages. In addition, recently achieved progress in nanomedicine opens a novel approach yielding from combination of modern nanotechnology with traditional treatment for innovative pharmacotherapeutics. In the present study, we investigated the antiaging effect of a pretreatment with Myrtus communis natural extract combined with a polycaprolactone nanofibrous scaffold (NanoPCL-M) on skin cell populations exposed to UV. We set up a novel model of skin on a bioreactor mimicking a crosstalk between keratinocytes, stem cells and fibroblasts, as in skin. Beta-galactosidase assay, indicating the amount of senescent cells, and viability assay, revealed that fibroblasts and stem cells pretreated with NanoPCL-M and then exposed to UV are superimposable to control cells, untreated and unexposed to UV damage. On the other hand, cells only exposed to UV stress, without NanoPCL-M pretreatment, exhibited a significantly higher yield of senescent elements. Keratinocyte-based 3D structures appeared disjointed after UV-stress, as compared to NanoPCL-M pretreated samples. Gene expression analysis performed on different senescence associated genes, revealed the activation of a molecular program of rejuvenation in stem cells pretreated with NanoPCL-M and then exposed to UV. Altogether, our results highlight a future translational application of NanoPCL-M to prevent skin aging.
Keywords: 4D dynamic model; biophysics; cell senescence; cellular mechanisms; nanofibers; natural extracts; precision medicine; skin aging; stem cells.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

