Exogenous ACC Deaminase Is Key to Improving the Performance of Pasture Legume-Rhizobial Symbioses in the Presence of a High Manganese Concentration
- PMID: 33255180
- PMCID: PMC7760732
- DOI: 10.3390/plants9121630
Exogenous ACC Deaminase Is Key to Improving the Performance of Pasture Legume-Rhizobial Symbioses in the Presence of a High Manganese Concentration
Abstract
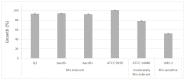
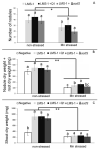
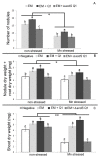
Manganese (Mn) toxicity is a very common soil stress around the world, which is responsible for low soil fertility. This manuscript evaluates the effect of the endophytic bacterium Pseudomonas sp. Q1 on different rhizobial-legume symbioses in the absence and presence of Mn toxicity. Three legume species, Cicer arietinum (chickpea), Trifolium subterraneum (subterranean clover), and Medicago polymorpha (burr medic) were used. To evaluate the role of 1-aminocyclopropane-1-carboxylate (ACC) deaminase produced by strain Q1 in these interactions, an ACC deaminase knockout mutant of this strain was constructed and used in those trials. The Q1 strain only promoted the symbiotic performance of Rhizobium leguminosarum bv. trifolii ATCC 14480T and Ensifer meliloti ATCC 9930T, leading to an increase of the growth of their hosts in both conditions. Notably, the acdS gene disruption of strain Q1 abolished the beneficial effect of this bacterium as well as causing this mutant strain to act deleteriously in those specific symbioses. This study suggests that the addition of non-rhizobia with functional ACC deaminase may be a strategy to improve the pasture legume-rhizobial symbioses, particularly when the use of rhizobial strains alone does not yield the expected results due to their difficulty in competing with native strains or in adapting to inhibitory soil conditions.
Keywords: abiotic stress; acidity; endophytes; nodulation; plant growth promotion; plant growth-promoting traits; plant–bacteria interactions.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Kochian L.V., Piñeros M.A., Hoekenga O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil. 2005;274:175–195. doi: 10.1007/s11104-004-1158-7. - DOI
-
- Gupta N., Gaurav S., Kumar A. Molecular basis of aluminium toxicity in plants: A review. Am. J. Plant Sci. 2013;4:21–37. doi: 10.4236/ajps.2013.412A3004. - DOI
-
- Bose J., Babourina O., Ma Y., Zhou M., Shabala S., Rengel Z. Specificity of ion uptake and homeostasis maintenance during acid and aluminium stresses. In: Panda S., Baluška F., editors. Aluminum Stress Adaptation in Plants. Signaling and Communication in Plants. Volume 24. Springer; Cham, Switzerland: 2015. pp. 229–251.
-
- von Uexküll H.R., Mutert E. Global extent, development and economic impact of acid soils. Plant Soil. 1995;171:1–15. doi: 10.1007/BF00009558. - DOI
Grants and funding
- POCI-01-0145-FEDER-016810 (PTDC/AGR-PRO/2978/2014)/FEDER - Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 - Operacional Programme for Competitiveness and Internationalisation (POCI)
- POCI-01-0145-FEDER-016810 (PTDC/AGR-PRO/2978/2014)/FCT - Fundação para a Ciência e a Tecnologia
- SFRH/BPD/94751/2013/FCT - Fundação para a Ciência
- UIDB/05183/2020/FCT - Fundação para a Ciência e a Tecnologia
- CEECIND/00093/2018/FCT - Fundação para a Ciência e a Tecnologia
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

