Sensitization of Cutaneous Primary Afferents in Bone Cancer Revealed by In Vivo Calcium Imaging
- PMID: 33255209
- PMCID: PMC7760605
- DOI: 10.3390/cancers12123491
Sensitization of Cutaneous Primary Afferents in Bone Cancer Revealed by In Vivo Calcium Imaging
Abstract
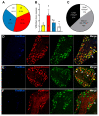
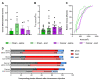
Cancer-induced bone pain (CIBP) is a complex condition, comprising components of inflammatory and neuropathic processes, but changes in the physiological response profiles of bone-innervating and cutaneous afferents remain poorly understood. We used a combination of retrograde labelling and in vivo calcium imaging of bone marrow-innervating dorsal root ganglia (DRG) neurons to determine the contribution of these cells in the maintenance of CIBP. We found a majority of femoral bone afferent cell bodies in L3 dorsal root ganglia (DRG) that also express the sodium channel subtype Nav1.8-a marker of nociceptive neurons-and lack expression of parvalbumin-a marker for proprioceptive primary afferents. Surprisingly, the response properties of bone marrow afferents to both increased intraosseous pressure and acid were unchanged by the presence of cancer. On the other hand, we found increased excitability and polymodality of cutaneous afferents innervating the ipsilateral paw in cancer bearing animals, as well as a behavioural phenotype that suggests changes at the level of the DRG contribute to secondary hypersensitivity. This study demonstrates that cutaneous afferents at distant sites from the tumour bearing tissue contribute to mechanical hypersensitivity, highlighting these cells as targets for analgesia.
Keywords: CIBP; DRG; bone afferents; in vivo imaging; nociception; peripheral sensitization; secondary hypersensitivity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

