Magnetic Resonance Imaging Correlates of White Matter Gliosis and Injury in Preterm Fetal Sheep Exposed to Progressive Systemic Inflammation
- PMID: 33255257
- PMCID: PMC7727662
- DOI: 10.3390/ijms21238891
Magnetic Resonance Imaging Correlates of White Matter Gliosis and Injury in Preterm Fetal Sheep Exposed to Progressive Systemic Inflammation
Abstract
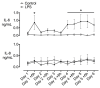
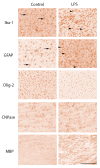
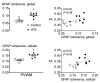
Progressive fetal infection/inflammation is strongly associated with neural injury after preterm birth. We aimed to test the hypotheses that progressively developing fetal inflammation leads to neuroinflammation and impaired white matter development and that the histopathological changes can be detected using high-field diffusion tensor magnetic resonance imaging (MRI). Chronically instrumented preterm fetal sheep at 0.7 of gestation were randomly assigned to receive intravenous saline (control; n = 6) or a progressive infusion of lipopolysaccharide (LPS, 200 ng intravenous over 24 h then doubled every 24 h for 5 days to induce fetal inflammation, n = 7). Sheep were killed 10 days after starting the infusions, for histology and high-field diffusion tensor MRI. Progressive LPS infusion was associated with increased circulating interleukin (IL)-6 concentrations and moderate increases in carotid artery perfusion and the frequency of electroencephalogram (EEG) activity (p < 0.05 vs. control). In the periventricular white matter, fractional anisotropy (FA) was increased, and orientation dispersion index (ODI) was reduced (p < 0.05 vs. control for both). Histologically, in the same brain region, LPS infusion increased microglial activation and astrocyte numbers and reduced the total number of oligodendrocytes with no change in myelination or numbers of immature/mature oligodendrocytes. Numbers of astrocytes in the periventricular white matter were correlated with increased FA and reduced ODI signal intensities. Astrocyte coherence was associated with increased FA. Moderate astrogliosis, but not loss of total oligodendrocytes, after progressive fetal inflammation can be detected with high-field diffusion tensor MRI.
Keywords: MRI; brain; infection; inflammation; preterm infant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Tumor necrosis factor inhibition attenuates white matter gliosis after systemic inflammation in preterm fetal sheep.J Neuroinflammation. 2020 Mar 23;17(1):92. doi: 10.1186/s12974-020-01769-6. J Neuroinflammation. 2020. PMID: 32293473 Free PMC article.
-
Interleukin-1 blockade attenuates white matter inflammation and oligodendrocyte loss after progressive systemic lipopolysaccharide exposure in near-term fetal sheep.J Neuroinflammation. 2021 Aug 31;18(1):189. doi: 10.1186/s12974-021-02238-4. J Neuroinflammation. 2021. PMID: 34465372 Free PMC article.
-
Magnesium sulphate reduces tertiary gliosis but does not improve EEG recovery or white or grey matter cell survival after asphyxia in preterm fetal sheep.J Physiol. 2023 May;601(10):1999-2016. doi: 10.1113/JP284381. Epub 2023 Apr 13. J Physiol. 2023. PMID: 36999348 Free PMC article.
-
Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white-matter injury.J Child Neurol. 2006 Jul;21(7):582-9. doi: 10.1177/08830738060210070101. J Child Neurol. 2006. PMID: 16970848 Review.
-
Putative Role of AMPK in Fetal Adaptive Brain Shut-Down: Linking Metabolism and Inflammation in the Brain.Front Neurol. 2014 Aug 11;5:150. doi: 10.3389/fneur.2014.00150. eCollection 2014. Front Neurol. 2014. PMID: 25157238 Free PMC article. Review. No abstract available.
Cited by
-
Increased Prostaglandin E2 in Brainstem Respiratory Centers Is Associated With Inhibition of Breathing Movements in Fetal Sheep Exposed to Progressive Systemic Inflammation.Front Physiol. 2022 Mar 3;13:841229. doi: 10.3389/fphys.2022.841229. eCollection 2022. Front Physiol. 2022. PMID: 35309054 Free PMC article.
-
Key roles of glial cells in the encephalopathy of prematurity.Glia. 2024 Mar;72(3):475-503. doi: 10.1002/glia.24474. Epub 2023 Nov 1. Glia. 2024. PMID: 37909340 Free PMC article. Review.
-
Alteration of the Oligodendrocyte Lineage Varies According to the Systemic Inflammatory Stimulus in Animal Models That Mimic the Encephalopathy of Prematurity.Front Physiol. 2022 Jul 19;13:881674. doi: 10.3389/fphys.2022.881674. eCollection 2022. Front Physiol. 2022. PMID: 35928559 Free PMC article. Review.
-
The synergistic effects of mechanical ventilation and intrauterine inflammation on cerebral inflammation in preterm fetal sheep.Front Cell Neurosci. 2024 Jun 19;18:1397658. doi: 10.3389/fncel.2024.1397658. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38962513 Free PMC article.
-
Postnatal cytomegalovirus infections did not alter amplitude-integrated electroencephalography signals or general movements in preterm infants.Acta Paediatr. 2025 Feb;114(2):332-339. doi: 10.1111/apa.17430. Epub 2024 Sep 20. Acta Paediatr. 2025. PMID: 39300940 Free PMC article.
References
-
- Honeycutt A., Dunlap L., Chen H., Al Homsi G., Grosse S., Schendel D. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment: United States, 2003. MMWR Morb. Mortal. Wkly. Rep. 2004;53:57–59. - PubMed
-
- Kuban K.C., Joseph R.M., O’Shea T.M., Heeren T., Fichorova R.N., Douglass L., Jara H., Frazier J.A., Hirtz D., Rollins J.V., et al. Extremely Low Gestational Age Newborns (ELGAN) Study Investigators, Circulating inflammatory-associated proteins in the first month of life and cognitive impairment at age 10 years in children born extremely preterm. J. Pediatr. 2017;180:116–123.e1. doi: 10.1016/j.jpeds.2016.09.054. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

