Behaviour of Human Oral Epithelial Cells Grown on Invisalign® SmartTrack® Material
- PMID: 33255259
- PMCID: PMC7727678
- DOI: 10.3390/ma13235311
Behaviour of Human Oral Epithelial Cells Grown on Invisalign® SmartTrack® Material
Abstract
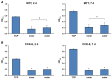
Invisalign aligners have been widely used to correct malocclusions, but their effect on oral cells is poorly known. Previous research evaluated the impact of aligners' eluates on various cells, but the cell behavior in direct contact with aligners is not yet studied. In the present study, we seeded oral epithelial cells (cell line Ca9-22) directly on Invisalign SmartTrack material. This material is composed of polyurethane and co-polyester and exhibit better mechanical characteristics compared to the predecessor. Cell morphology and behavior were investigated by scanning electron microscopy and an optical cell moves analyzer. The effect of aligners on cell proliferation/viability was assessed by cell-counting kit (CCK)-8 and 3,4,5-dimethylthiazol-2-yl-2,5-diphenyl tetrazolium bromide (MTT) assay and live/dead staining. The expression of inflammatory markers and proteins involved in epithelial barrier function was measured by qPCR. Cells formed cluster-like structures on aligners. The proliferation/viability of cells growing on aligners was significantly lower (p < 0.05) compared to those growing on tissue culture plastic (TCP). Live/dead staining revealed a rare occurrence of dead cells on aligners. The gene expression level of all inflammatory markers in cells grown on aligners' surfaces was significantly increased (p < 0.05) compared to cells grown on TCP after two days. Gene expression levels of the proteins involved in barrier function significantly increased (p < 0.05) on aligners' surfaces after two and seven days of culture. Aligners' material exhibits no cytotoxic effect on oral epithelial cells, but alters their behavior and the expression of proteins involved in the inflammatory response, and barrier function. The clinical relevance of these effects has still to be established.
Keywords: Invisalign; SmartTrack; aligner; epithelial barrier; in vitro; inflammation; oral epithelial cells; orthodontics; proliferation.
Conflict of interest statement
All authors declare no conflict of interest.
Figures






References
-
- Condo R., Pazzini L., Cerroni L., Pasquantonio G., Lagana G., Pecora A., Mussi V., Rinaldi A., Mecheri B., Licoccia S., et al. Mechanical properties of “two generations” of teeth aligners: Change analysis during oral permanence. Dent. Mater. J. 2018;37:835–842. doi: 10.4012/dmj.2017-323. - DOI - PubMed
LinkOut - more resources
Full Text Sources

