TrkB-Targeted Therapy for Mucoepidermoid Carcinoma
- PMID: 33255325
- PMCID: PMC7759804
- DOI: 10.3390/biomedicines8120531
TrkB-Targeted Therapy for Mucoepidermoid Carcinoma
Abstract
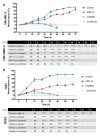
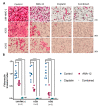
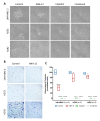
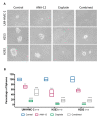
The brain-derived neurotrophic factor (BDNF)/tyrosine receptor kinase B (TrkB) pathway was previously associated with key oncogenic outcomes in a number of adenocarcinomas. The aim of our study was to determine the role of this pathway in mucoepidermoid carcinoma (MEC). Three MEC cell lines (UM-HMC-2, H253 and H292) were exposed to Cisplatin, the TrkB inhibitor, ANA-12 and a combination of these drugs. Ultrastructural changes were assessed through transmission electron microscopy; scratch and Transwell assays were used to assess migration and invasion; and a clonogenic assay and spheroid-forming assay allowed assessment of survival and percentage of cancer stem cells (CSC). Changes in cell ultrastructure demonstrated Cisplatin cytotoxicity, while the effects of ANA-12 were less pronounced. Both drugs, used individually and in combination, delayed MEC cell migration, invasion and survival. ANA-12 significantly reduced the number of CSC, but the Cisplatin effect was greater, almost eliminating this cell population in all MEC cell lines. Interestingly, the spheroid forming capacity recovered, following the combination therapy, as compared to Cisplatin alone. Our studies allowed us to conclude that the TrkB inhibition, efficiently impaired MEC cell migration, invasion and survival in vitro, however, the decrease in CSC number, following the combined treatment of ANA-12 and Cisplatin, was less than that seen with Cisplatin alone; this represents a limiting factor.
Keywords: adenocarcinoma; cell biology; head and neck neoplasms; salivary gland neoplasms; therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Observatory: Cancer Tomorrow. International Agency for Research on Cancer; Lyon, France: 2018. [(accessed on 5 February 2020)]. Available online: https://gco.iarc.fr/tomorrow.
-
- El-Naggar A.K., Chan J.K.C., Grandis J.R., Takata T., Slootweg P.J. WHO Classification of Head and Neck Tumours. 4th ed. Volume 9 IARC; Lyon, France: 2017.
-
- Fonseca F.P., Carvalho Mde V., de Almeida O.P., Rangel A.L., Takizawa M.C., Bueno A.G., Vargas P.A. Clinicopathologic analysis of 493 cases of salivary gland tumours in a Southern Brazilian population. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012;114:230–239. doi: 10.1016/j.oooo.2012.04.008. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

