Effects of Fecal Microbiota Transplantation on Composition in Mice with CKD
- PMID: 33255454
- PMCID: PMC7761367
- DOI: 10.3390/toxins12120741
Effects of Fecal Microbiota Transplantation on Composition in Mice with CKD
Abstract
Background: Chronic kidney disease (CKD) is a renal disorder characterized by the accumulation of uremic toxins with limited strategies to reduce their concentrations. A large amount of data supports the pivotal role of intestinal microbiota in CKD complications and as a major source of uremic toxins production. Here, we explored whether fecal microbiota transplantation (FMT) could be attenuated in metabolic complication and uremic toxin accumulation in mice with CKD.
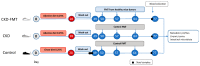
Methods: Kidney failure was chemically induced by a diet containing 0.25% (w/w) of adenine for four weeks. Mice were randomized into three groups: control, CKD and CKD + FMT groups. After four weeks, CKD mice underwent fecal microbiota transplantation (FMT) from healthy mice or phosphate buffered saline as control. The gut microbiota structure, uremic toxins plasmatic concentrations, and metabolic profiles were explored three weeks after transplantation.
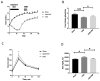
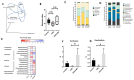
Results: Associated with the increase of alpha diversity, we observed a noticeable improvement of gut microbiota disturbance, after FMT treatment. FMT further decreased p-cresyl sulfate accumulation and improved glucose tolerance. There was no change in kidney function.
Conclusions: These data indicate that FMT limited the accumulation of uremic toxins issued from intestinal cresol pathway by a beneficial effect on gut microbiota diversity. Further studies are needed to investigate the FMT efficiency, the timing and feces amount for the transplantation before, to become a therapeutic option in CKD patients.
Keywords: chronic kidney disease; fecal microbiota transplantation; p-cresyl-sulfate; uremic toxins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

