Role of PB1 Midbody Remnant Creating Tethered Polar Bodies during Meiosis II
- PMID: 33255457
- PMCID: PMC7760350
- DOI: 10.3390/genes11121394
Role of PB1 Midbody Remnant Creating Tethered Polar Bodies during Meiosis II
Abstract
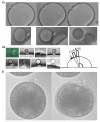
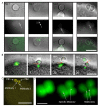
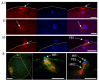
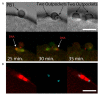
Polar body (PB) formation is an extreme form of unequal cell division that occurs in oocytes due to the eccentric position of the small meiotic spindle near the oocyte cortex. Prior to PB formation, a chromatin-centered process causes the cortex overlying the meiotic chromosomes to become polarized. This polarized cortical subdomain marks the site where a cortical protrusion or outpocket forms at the oocyte surface creating the future PBs. Using ascidians, we observed that PB1 becomes tethered to the fertilized egg via PB2, indicating that the site of PB1 cytokinesis directed the precise site for PB2 emission. We therefore studied whether the midbody remnant left behind following PB1 emission was involved, together with the egg chromatin, in defining the precise cortical site for PB2 emission. During outpocketing of PB2 in ascidians, we discovered that a small structure around 1 µm in diameter protruded from the cortical outpocket that will form the future PB2, which we define as the "polar corps". As emission of PB2 progressed, this small polar corps became localized between PB2 and PB1 and appeared to link PB2 to PB1. We tested the hypothesis that this small polar corps on the surface of the forming PB2 outpocket was the midbody remnant from the previous round of PB1 cytokinesis. We had previously discovered that Plk1::Ven labeled midbody remnants in ascidian embryos. We therefore used Plk1::Ven to follow the dynamics of the PB1 midbody remnant during meiosis II. Plk1::Ven strongly labeled the small polar corps that formed on the surface of the cortical outpocket that created PB2. Following emission of PB2, this polar corps was rich in Plk1::Ven and linked PB2 to PB1. By labelling actin (with TRITC-Phalloidin) we also demonstrated that actin accumulates at the midbody remnant and also forms a cortical cap around the midbody remnant in meiosis II that prefigured the precise site of cortical outpocketing during PB2 emission. Phalloidin staining of actin and immunolabelling of anti-phospho aPKC during meiosis II in fertilized eggs that had PB1 removed suggested that the midbody remnant remained within the fertilized egg following emission of PB1. Dynamic imaging of microtubules labelled with Ens::3GFP, MAP7::GFP or EB3::3GFP showed that one pole of the second meiotic spindle was located near the midbody remnant while the other pole rotated away from the cortex during outpocketing. Finally, we report that failure of the second meiotic spindle to rotate can lead to the formation of two cortical outpockets at anaphase II, one above each set of chromatids. It is not known whether the midbody remnant of PB1 is involved in directing the precise location of PB2 since our data are correlative in ascidians. However, a review of the literature indicates that PB1 is tethered to the egg surface via PB2 in several species including members of the cnidarians, lophotrochozoa and echinoids, suggesting that the midbody remnant formed during PB1 emission may be involved in directing the precise site of PB2 emission throughout the invertebrates.
Keywords: ascidian; meiotic spindle; midbody remnant; second polar body.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Eager D.D., Johnson M.H., Thurley K.W. Ultrastructural studies on the surface membrane of the mouse egg. J. Cell Sci. 1976;22:345–353. - PubMed
-
- Maro B., Johnson M.H., Pickering S.J., Flach G. Changes in actin distribution during fertilization of the mouse egg. J. Embryol. Exp. Morphol. 1984;81:211–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

