Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of PANI-PEDOT Film Deposited on Transparent Electrode
- PMID: 33255495
- PMCID: PMC7761354
- DOI: 10.3390/polym12122778
Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of PANI-PEDOT Film Deposited on Transparent Electrode
Abstract
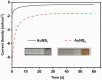
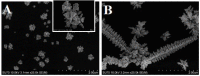
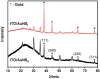
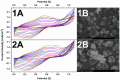
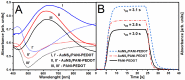
Conjugated polymers (CPs) are attractive materials for use in different areas; nevertheless, the enhancement of electrochromic stability and switching time is still necessary to expand the commercialization of electrochromic devices. To our best knowledge, this is the first study demonstrating the employment of electrodeposited gold nanostructures (AuNS) for the enhancement of CPs' electrochromic properties when a transparent electrode is used as a substrate. Polyaniline-poly(3,4-ethylenedioxythiophene) (PANI-PEDOT) films were electrodeposited on a transparent indium tin oxide glass electrode, which was pre-modified by two different methods. AuNS were electrodeposited at -0.2 V constant potential for 60 s using both the 1st method (synthesis solution consisted of 3 mM HAuCl4 and 0.1 M H2SO4) and 2nd method (15 mM HAuCl4 and 1 M KNO3) resulting in an improvement of optical contrast by 3% and 22%, respectively. Additionally, when using the 1st method, the coloration efficiency was improved by 50% while the switching time was reduced by 17%. Furthermore, in both cases, the employment of AuNS resulted in an enhancement of the electrochromic stability of the CPs layer. A further selection of AuNS pre-modification conditions with the aim to control their morphology and size can be a possible stepping stone for the further improvement of CPs electrochromic properties.
Keywords: conducting polymers; electrochromic polymers; gold nanostructures; poly(3,4-ethylenedioxythiophene); polyaniline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Deshmukh M.A., Gicevicius M., Ramanaviciene A., Shirsat M.D., Viter R., Ramanavicius A. Hybrid electrochemical/electrochromic Cu(II) ion sensor prototype based on PANI/ITO-electrode. Sens. Actuators B Chem. 2017;248:527–535. doi: 10.1016/j.snb.2017.03.167. - DOI
-
- Popov A., Brasiunas B., Mikoliunaite L., Bagdziunas G., Ramanavicius A., Ramanaviciene A. Comparative study of polyaniline (PANI), poly(3,4-ethylenedioxythiophene) (PEDOT) and PANI-PEDOT films electrochemically deposited on transparent indium thin oxide based electrodes. Polymer. 2019;172:133–141. doi: 10.1016/j.polymer.2019.03.059. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

