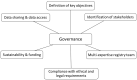
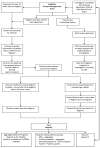
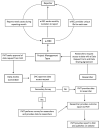
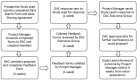
The EuRRECa Project as a Model for Data Access and Governance Policies for Rare Disease Registries That Collect Clinical Outcomes
- PMID: 33255540
- PMCID: PMC7727867
- DOI: 10.3390/ijerph17238743
The EuRRECa Project as a Model for Data Access and Governance Policies for Rare Disease Registries That Collect Clinical Outcomes
Abstract
Rare disease (RD) registries are important platforms that facilitate communication between health care professionals, patients and other members of the multidisciplinary team. RD registries enable data sharing and promotion of research and audits, often in an international setting, with the overall aim of improving patient care. RD registries also have a fundamental role in supporting the work of clinical networks such as the European Reference Networks (ERNs) for rare diseases. With the recent expansion of RD registries, it has become even more essential to outline standards of good practice in relation to governance, infrastructure, documentation, training, audits and adopting the Findable, Accessible, Interoperable and Reusable (FAIR) data principles to maintain registries of high quality. For the purpose of this paper, we highlight vital aspects of data access and data governance policies for RD registries, using the European Registries for Rare Endocrine Conditions (EuRRECa) as an example of a project that aims to promote good standards of practice for improving the quality of utilization of RD registries.
Keywords: European reference networks; databases; endocrinology; rare conditions; rare diseases; registries.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Recommendations for Improving the Quality of Rare Disease Registries.Int J Environ Res Public Health. 2018 Aug 3;15(8):1644. doi: 10.3390/ijerph15081644. Int J Environ Res Public Health. 2018. PMID: 30081484 Free PMC article.
-
Supporting international networks through platforms for standardised data collection-the European Registries for Rare Endocrine Conditions (EuRRECa) model.Endocrine. 2021 Mar;71(3):555-560. doi: 10.1007/s12020-021-02617-0. Epub 2021 Jan 29. Endocrine. 2021. PMID: 33512655 Free PMC article. Review.
-
The Quality Evaluation of Rare Disease Registries-An Assessment of the Essential Features of a Disease Registry.Int J Environ Res Public Health. 2021 Nov 15;18(22):11968. doi: 10.3390/ijerph182211968. Int J Environ Res Public Health. 2021. PMID: 34831724 Free PMC article.
-
National registries of rare diseases in Europe: an overview of the current situation and experiences.Public Health Genomics. 2015;18(1):20-5. doi: 10.1159/000365897. Epub 2014 Sep 9. Public Health Genomics. 2015. PMID: 25228300
-
The Role of the European Reference Network for Rare Bone Diseases (ERN BOND) and European Registries for Rare Bone and Mineral Conditions (EuRR-Bone) in the Governance of the Management of Rare Bone and Mineral Diseases.Calcif Tissue Int. 2024 Nov;115(5):498-506. doi: 10.1007/s00223-024-01256-7. Epub 2024 Jul 26. Calcif Tissue Int. 2024. PMID: 39060404 Free PMC article. Review.
Cited by
-
Hereditary Endocrine Tumor Registries.J Endocr Soc. 2022 Dec 23;7(3):bvac194. doi: 10.1210/jendso/bvac194. eCollection 2023 Jan 6. J Endocr Soc. 2022. PMID: 36632485 Free PMC article.
-
A systematic overview of rare disease patient registries: challenges in design, quality management, and maintenance.Orphanet J Rare Dis. 2023 May 5;18(1):106. doi: 10.1186/s13023-023-02719-0. Orphanet J Rare Dis. 2023. PMID: 37147718 Free PMC article. Review.
-
Data collection on rare bone and mineral conditions in Europe: The landscape of registries and databases.Eur J Med Genet. 2023 Dec;66(12):104868. doi: 10.1016/j.ejmg.2023.104868. Epub 2023 Oct 30. Eur J Med Genet. 2023. PMID: 38832910 Free PMC article.
-
Development and implementation of the AIDA International Registry for patients with Periodic Fever, Aphthous stomatitis, Pharyngitis, and cervical Adenitis syndrome.Front Pediatr. 2022 Jul 22;10:930305. doi: 10.3389/fped.2022.930305. eCollection 2022. Front Pediatr. 2022. PMID: 35935379 Free PMC article.
-
Development and Implementation of the AIDA International Registry for Patients With Still's Disease.Front Med (Lausanne). 2022 Apr 7;9:878797. doi: 10.3389/fmed.2022.878797. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35463015 Free PMC article.
References
-
- Regulation (EC) No 141/2000 of the European Parliament and of the Council of 16 December 1999 on Orphan Medicinal Products. [(accessed on 26 August 2020)]; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32000R0141.
-
- EURCERD Core Recommendations on Rare Disease Patient Registration and Data Collection. [(accessed on 2 August 2020)]; Available online: http://www.eucerd.eu/wp-content/uploads/2013/06/EUCERD_Recommendations_R....
-
- Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the Application of Patients’ Rights in Cross-Border Healthcare. Off. J. Eur. Union. 2011;L88:45–65.
-
- Orphanet Report Series-Rare Disease Registries in Europe—May 2019. [(accessed on 23 August 2020)]; Available online: https://www.orpha.net/orphacom/cahiers/docs/GB/Registries.pdf.
-
- Kodra Y., Weinback J., Posada-de-la-Paz M., Coi A., Lemonnier S.L., van Enckvort D., Roos M., Jacobson A., Cornet R., Ahmed S.F., et al. Recommendations for improving the quality of rare disease registries. Int. J. Environ. Res. Public Health. 2018;15:1644. doi: 10.3390/ijerph15081644. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous