Optimization of Liposomes for Antigen Targeting to Splenic CD169+ Macrophages
- PMID: 33255564
- PMCID: PMC7760819
- DOI: 10.3390/pharmaceutics12121138
Optimization of Liposomes for Antigen Targeting to Splenic CD169+ Macrophages
Abstract
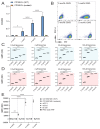
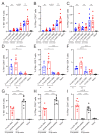
Despite promising progress in cancer vaccination, therapeutic effectiveness is often insufficient. Cancer vaccine effectiveness could be enhanced by targeting vaccine antigens to antigen-presenting cells, thereby increasing T-cell activation. CD169-expressing splenic macrophages efficiently capture particulate antigens from the blood and transfer these antigens to dendritic cells for the activation of CD8+ T cells. In this study, we incorporated a physiological ligand for CD169, the ganglioside GM3, into liposomes to enhance liposome uptake by CD169+ macrophages. We assessed how variation in the amount of GM3, surface-attached PEG and liposomal size affected the binding to, and uptake by, CD169+ macrophages in vitro and in vivo. As a proof of concept, we prepared GM3-targeted liposomes containing a long synthetic ovalbumin peptide and tested the capacity of these liposomes to induce CD8+ and CD4+ T-cell responses compared to control liposomes or soluble peptide. The data indicate that the delivery of liposomes to splenic CD169+ macrophages can be optimized by the selection of liposomal constituents and liposomal size. Moreover, optimized GM3-mediated liposomal targeting to CD169+ macrophages induces potent immune responses and therefore presents as an interesting delivery strategy for cancer vaccination.
Keywords: CD169; GM3; Siglec-1; T cells; cancer vaccination; liposome; macrophage; sialoadhesin; targeting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

