Simultaneous Detection of CNVs and SNVs Improves the Diagnostic Yield of Fetuses with Ultrasound Anomalies and Normal Karyotypes
- PMID: 33255631
- PMCID: PMC7759943
- DOI: 10.3390/genes11121397
Simultaneous Detection of CNVs and SNVs Improves the Diagnostic Yield of Fetuses with Ultrasound Anomalies and Normal Karyotypes
Abstract
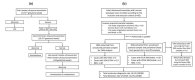
The routine assessment to determine the genetic etiology for fetal ultrasound anomalies follows a sequential approach, which usually takes about 6-8 weeks turnaround time (TAT). We evaluated the clinical utility of simultaneous detection of copy number variations (CNVs) and single nucleotide variants (SNVs)/small insertion-deletions (indels) in fetuses with a normal karyotype with ultrasound anomalies. We performed CNV detection by chromosomal microarray analysis (CMA) or low pass CNV-sequencing (CNV-seq), and in parallel SNVs/indels detection by trio-based clinical exome sequencing (CES) or whole exome sequencing (WES). Eight-three singleton pregnancies with a normal fetal karyotype were enrolled in this prospective observational study. Pathogenic or likely pathogenic variations were identified in 30 cases (CNVs in 3 cases, SNVs/indels in 27 cases), indicating an overall molecular diagnostic rate of 36.1% (30/83). Two cases had both a CNV of uncertain significance (VOUS) and likely pathogenic SNV, and one case carried both a VOUS CNV and an SNV. We demonstrated that simultaneous analysis of CNVs and SNVs/indels can improve the diagnostic yield of prenatal diagnosis with shortened reporting time, namely, 2-3 weeks. Due to the relatively long TAT for sequential procedure for prenatal genetic diagnosis, as well as recent sequencing technology advancements, it is clinically necessary to consider the simultaneous evaluation of CNVs and SNVs/indels to enhance the diagnostic yield and timely TAT, especially for cases in the late second trimester or third trimester.
Keywords: CMA; CNV-seq; clinical exome sequencing (CES); fetal ultrasound anomalies; prenatal diagnosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Clinical utility of trio whole exome sequencing in fetuses with ultrasound anomalies.Hum Genomics. 2025 Apr 5;19(1):37. doi: 10.1186/s40246-025-00745-6. Hum Genomics. 2025. PMID: 40188065 Free PMC article.
-
The Value of a Comprehensive Genomic Evaluation in Prenatal Diagnosis of Genetic Diseases: A Retrospective Study.Genes (Basel). 2022 Dec 14;13(12):2365. doi: 10.3390/genes13122365. Genes (Basel). 2022. PMID: 36553632 Free PMC article.
-
Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): a cohort study.Lancet. 2019 Feb 23;393(10173):747-757. doi: 10.1016/S0140-6736(18)31940-8. Epub 2019 Jan 31. Lancet. 2019. PMID: 30712880 Free PMC article.
-
Prenatal diagnosis in the fetal hyperechogenic kidneys: assessment using chromosomal microarray analysis and exome sequencing.Hum Genet. 2023 Jun;142(6):835-847. doi: 10.1007/s00439-023-02545-1. Epub 2023 Apr 24. Hum Genet. 2023. PMID: 37095353 Review.
-
Prenatal Genome-Wide Sequencing analysis (Exome or Genome) in detecting pathogenic Single Nucleotide Variants in fetal Central Nervous System Anomalies: systematic review and meta-analysis.Eur J Hum Genet. 2024 Jul;32(7):759-769. doi: 10.1038/s41431-024-01590-2. Epub 2024 Mar 15. Eur J Hum Genet. 2024. PMID: 38486024 Free PMC article.
Cited by
-
Expanding the mutational spectrum of ZTTK syndrome: A de novo variant with global developmental delay and malnutrition in a Chinese patient.Mol Genet Genomic Med. 2023 Aug;11(8):e2188. doi: 10.1002/mgg3.2188. Epub 2023 Jul 24. Mol Genet Genomic Med. 2023. PMID: 37488749 Free PMC article. Review.
-
Small size, big problems: insights and difficulties in prenatal diagnosis of fetal microcephaly.Front Neurosci. 2024 Mar 12;18:1347506. doi: 10.3389/fnins.2024.1347506. eCollection 2024. Front Neurosci. 2024. PMID: 38533444 Free PMC article. Review.
-
PIEZO1 variant implications for biological understanding and human health.Open Biol. 2025 Jul;15(7):240345. doi: 10.1098/rsob.240345. Epub 2025 Jul 9. Open Biol. 2025. PMID: 40628291 Free PMC article. Review.
-
A method to comprehensively identify germline SNVs, INDELs and CNVs from whole exome sequencing data of BRCA1/2 negative breast cancer patients.NAR Genom Bioinform. 2024 Apr 17;6(2):lqae033. doi: 10.1093/nargab/lqae033. eCollection 2024 Jun. NAR Genom Bioinform. 2024. PMID: 38633426 Free PMC article.
-
Prenatal Diagnosis and Outcomes in Fetuses with Hemivertebra.Genes (Basel). 2022 Sep 9;13(9):1623. doi: 10.3390/genes13091623. Genes (Basel). 2022. PMID: 36140791 Free PMC article.
References
-
- Lord J., McMullan D.J., Eberhardt R.Y., Rinck G., Hamilton S.J., Quinlan-Jones E., Prigmore E., Keelagher R., Best S.K., Carey G.K., et al. Prenatal exome sequencing analysis in fetal structural anomalies detected by ultrasonography (PAGE): A cohort study. Lancet. 2019;393:747–757. doi: 10.1016/S0140-6736(18)31940-8. - DOI - PMC - PubMed
-
- Petrovski S., Aggarwal V., Giordano J.L., Stosic M., Wou K., Bier L., Spiegel E., Brennan K., Stong N., Jobanputra V., et al. Whole-exome sequencing in the evaluation of fetal structural anomalies: A prospective cohort study. Lancet. 2019;393:758–767. doi: 10.1016/S0140-6736(18)32042-7. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

