Pharmacokinetics of Sodium and Calcium Salts of (6S)-5-Methyltetrahydrofolic Acid Compared to Folic Acid and Indirect Comparison of the Two Salts
- PMID: 33255787
- PMCID: PMC7760477
- DOI: 10.3390/nu12123623
Pharmacokinetics of Sodium and Calcium Salts of (6S)-5-Methyltetrahydrofolic Acid Compared to Folic Acid and Indirect Comparison of the Two Salts
Abstract
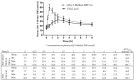
(6S)-5-Methyltetrahydrofolic acid ((6S)-5-Methyl-THF) salts and folic acid may differ in their abilities to raise plasma (6S)-5-Methyl-THF levels. We compared the area under the curve (AUC), Cmax, and Tmax of plasma (6S)-5-Methyl-THF after intakes of (6S)-5-Methyl-THF-Na salt (Arcofolin®) and folic acid. Moreover, we compared the AUCs after intakes of (6S)-5-Methyl-THF-Na and the calcium salt, (6S)-5-Methyl-THF-Ca, that were tested against folic acid in two independent studies. The study was randomized, double blind, and cross over. Twenty-four adults (12 men and 12 women) received a single oral dose of 436 µg (6S)-5-Methyl-THF-Na and an equimolar dose of folic acid (400 µg) on two kinetic days with two weeks washout period in between. The plasma concentrations of (6S)-5-Methyl-THF were measured at 9 time points between 0 and 8 h. We found that the AUC0-8 h of plasma (6S)-5-Methyl-THF (mean (SD) = 126.0 (33.6) vs. 56.0 (25.3) nmol/L*h) and Cmax (36.8 (10.8) vs. 11.1 (4.1) nmol/L) were higher after administration of (6S)-5-Methyl-THF-Na than after the administration of folic acid (p < 0.001 for both). These differences were present in men and women. Only administration of folic acid resulted in a transient increase in plasma unmetabolized folic acid (2.5 (2.0) nmol/L after 0.5 h and 4.7 (2.9) nmol/L after 1 h). Intake of (6S)-5-Methyl-THF-Na was safe. The ratios of the AUC0-8 h for (6S)-5-Methyl-THF-Na and (6S)-5-Methyl-THF-Ca to the corresponding folic acid reference group and the delta of these AUC0-8 h did not differ between the studies. In conclusion, a single oral dose of (6S)-5-Methyl-THF-Na caused higher AUC0-8 h and Cmax of plasma (6S)-5-Methyl-THF compared to folic acid. The Na- and Ca- salts of (6S)-5-Methyl-THF are not likely to differ in their pharmacokinetics. Further studies may investigate whether supplementation of the compounds for a longer time will lead to differences in circulating or intracellular/tissue folate concentrations.
Keywords: (6S)-5-Methyl-THF; Arcofolin®; Metafolin®; bioavailability; folic acid; homocysteine; plasma folate; red blood cell folate.
Conflict of interest statement
BioTeSys is an independent Nutritional CRO. R.O. served as external consultant during the study. M.W. is an independent statistician supporting data analysis.
Figures

Similar articles
-
Enhanced oral bioavailability of a novel folate salt: comparison with folic acid and a calcium folate salt in a pharmacokinetic study in rats.Minerva Ginecol. 2016 Apr;68(2):99-105. Minerva Ginecol. 2016. PMID: 27008238
-
Comparison of (6 S)-5-methyltetrahydrofolic acid v. folic acid as the reference folate in longer-term human dietary intervention studies assessing the relative bioavailability of natural food folates: comparative changes in folate status following a 16-week placebo-controlled study in healthy adults.Br J Nutr. 2010 Mar;103(5):724-9. doi: 10.1017/S0007114509992339. Epub 2009 Oct 26. Br J Nutr. 2010. PMID: 19852872 Clinical Trial.
-
Is natural (6S)-5-methyltetrahydrofolic acid as effective as synthetic folic acid in increasing serum and red blood cell folate concentrations during pregnancy? A proof-of-concept pilot study.Trials. 2020 May 5;21(1):380. doi: 10.1186/s13063-020-04320-3. Trials. 2020. PMID: 32370802 Free PMC article.
-
L-ascorbic acid improves the serum folate response to an oral dose of [6S]-5-methyltetrahydrofolic acid in healthy men.Eur J Clin Nutr. 2008 Oct;62(10):1224-30. doi: 10.1038/sj.ejcn.1602840. Epub 2007 Jul 11. Eur J Clin Nutr. 2008. PMID: 17622258 Clinical Trial.
-
Folic acid and L-5-methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics.Clin Pharmacokinet. 2010 Aug;49(8):535-48. doi: 10.2165/11532990-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20608755 Review.
Cited by
-
Innovative, rapid, high-throughput method for drug repurposing in a pandemic-A case study of SARS-CoV-2 and COVID-19.Front Pharmacol. 2023 Mar 1;14:1130828. doi: 10.3389/fphar.2023.1130828. eCollection 2023. Front Pharmacol. 2023. PMID: 36937851 Free PMC article.
-
Conversion of calcium-l-methylfolate and (6S)-5-methyltetrahydrofolic acid glucosamine salt into dietary folate equivalents.EFSA J. 2022 Aug 24;20(8):e07452. doi: 10.2903/j.efsa.2022.7452. eCollection 2022 Aug. EFSA J. 2022. PMID: 36034319 Free PMC article.
-
Safety of monosodium salt of l-5-methyltetrahydrofolic acid as a novel food pursuant to Regulation (EU) 2015/2283 and the bioavailability of folate from this source in the context of Directive 2002/46/EC, Regulation (EU) No 609/2013 and Regulation (EC) No 1925/2006.EFSA J. 2023 Nov 29;21(11):e8417. doi: 10.2903/j.efsa.2023.8417. eCollection 2023 Nov. EFSA J. 2023. PMID: 38035146 Free PMC article.
-
Human milk unmetabolized folic acid is increased following supplementation with synthetic folic acid as compared to (6S)-5-methyltetrahydrofolic acid.Sci Rep. 2023 Jul 12;13(1):11298. doi: 10.1038/s41598-023-38224-4. Sci Rep. 2023. PMID: 37438496 Free PMC article. Clinical Trial.
-
Serum 5-Methyltetrahydrofolate Status Is Associated with One-Carbon Metabolism-Related Metabolite Concentrations and Enzyme Activity Indicators in Young Women.Int J Mol Sci. 2023 Jul 1;24(13):10993. doi: 10.3390/ijms241310993. Int J Mol Sci. 2023. PMID: 37446171 Free PMC article.
References
-
- Veena S.R., Krishnaveni G.V., Srinivasan K., Wills A.K., Muthayya S., Kurpad A.V., Yajnik C.S., Fall C.H. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10- year-old children in South India. J. Nutr. 2010;140:1014–1022. doi: 10.3945/jn.109.118075. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

