Cerebrospinal Fluid Biomarkers in Relation to MRZ Reaction Status in Primary Progressive Multiple Sclerosis
- PMID: 33255854
- PMCID: PMC7761295
- DOI: 10.3390/cells9122543
Cerebrospinal Fluid Biomarkers in Relation to MRZ Reaction Status in Primary Progressive Multiple Sclerosis
Abstract
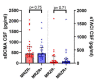
The MRZ reaction (MRZR) comprises the three antibody indices (AIs) against measles, rubella, and varicella zoster virus, reflecting an intrathecal polyspecific B cell response highly specific for multiple sclerosis (MS). Thus, MRZR can be used to confirm a diagnosis of primary progressive MS (PPMS) but its pathophysiological and wider clinical relevance is unclear. This study aimed to investigate whether PPMS patients with a positive MRZR (MRZR+) differ from those with a negative MRZR (MRZR-) according to cerebrospinal fluid (CSF) biomarkers of B cell activity, neuroaxonal damage or glial activity, and clinical features. (1) Methods: In a multicenter PPMS cohort (n = 81) with known MRZR status, we measured B cell-activating factor (BAFF), chemokine CXC ligand 13 (CXCL-13), soluble B cell maturation antigen (sBCMA), soluble transmembrane activator and CAML interactor (sTACI), and chitinase-3-like protein 1 (CHI3L1) in the CSF with enzyme-linked immunosorbent assays (ELISAs). Glial fibrillary acidic protein (GFAP) and neurofilament light chain (NfL) were detected in serum and CSF using single molecule array (SIMOA) technology. (2) Results: MRZR+ patients (45.7% of all PPMS patients) revealed higher levels of NfL in CSF compared to MRZR- patients (54.3%). There were positive correlations between each of sBCMA, sTACI, and intrathecal immunoglobin G (IgG) synthesis. Additionally, NfL concentrations in serum positively correlated with those in CSF and those of GFAP in serum. However, MRZR+ and MRZR- patients did not differ concerning clinical features (e.g., age, disease duration, Expanded Disability Status Scale (EDSS) at diagnosis and follow-up); CSF routine parameters; CSF concentrations of BAFF, CXCL-13, sBCMA, sTACI, CHI3L1, and GFAP; or serum concentrations of GFAP and NfL. (3) Conclusions: In PPMS patients, MRZR positivity might indicate a more pronounced axonal damage. Higher levels of the soluble B cell receptors BCMA and transmembrane activator and CAML interactor (TACI) in CSF are associated with a stronger intrathecal IgG synthesis in PPMS.
Keywords: B cell-activating factor (BAFF); MRZ reaction (MRZR); chemokine CXC ligand 13 (CXCL-13); chitinase-3-like protein 1 (CHI3L1); glial fibrillary acidic protein (GFAP); neurofilament light chain (NfL); primary progressive multiple sclerosis (PPMS); soluble B cell maturation antigen (sBCMA); soluble transmembrane activator and CAML interactor (sTACI).
Conflict of interest statement
T.R. received travel grants from Bayer Vital GmbH, Biogen Idec, and Novartis. A.A. received research funding from DMSG, travel grants from Biogen Idec, and institutional research grants from Merck Serono and Novartis, all outside the submitted study. T.B. has nothing to disclose. E.M. received honorarium from Roche, Novartis, Sanofi-Aventis, Biogen Idec, and Bioeq, and grant support from Novartis, Sanofi-Aventis, and Merck Serono. M.O. gave scientific advice for Axon, Roche, Fujirebio, and Biogen Idec. U.K.Z. received speaker fees, travel compensation, and/or his section received research support from Alexion, Almirall, Bayer Health Care, Biogen Idec, Celgene, Genzyme, Merck Serono, Novartis, Roche, Sanofi-Aventis, and Teva, and grants from the German Ministry for Education and Research (BMBF), German Ministry for Economy (BMWi), Deutsche Forschungsgemeinschaft (DFG), and European Union (EU), outside the submitted work. R.D. received lecture fees by Roche and travel grants from Biogen Idec. H.T. reports funding for research projects, lectures, and travel from Bayer Vital GmbH, Biogen Idec, Genzyme, Merck Serono, Novartis, Roche, and Teva, and received research support from DMSG and BMBF. S.R. reports receiving consulting and lecture fees, research grants, and research support from Baxter, Bayer Vital GmbH, Biogen Idec, Genzyme, Merck Serono, Novartis, R.G., Sanofi-Aventis, and Teva. S.R. indicates that he is a founding executive board member of ravo Diagnostika GmbH (Oltmannsstrasse 5, D-79100 Freiburg, Germany), which sells in vitro diagnostic medical devices for the detection of infectious diseases and paraneoplastic autoantibodies. A.H. has nothing to disclose.
Figures

Similar articles
-
Glial and neuroaxonal biomarkers in a multiple sclerosis (MS) cohort.Hell J Nucl Med. 2019 Sep-Dec;22 Suppl 2:113-121. Hell J Nucl Med. 2019. PMID: 31802051
-
The MRZ reaction and a quantitative intrathecal IgG synthesis may be helpful to differentiate between primary central nervous system lymphoma and multiple sclerosis.J Neurol. 2018 May;265(5):1106-1114. doi: 10.1007/s00415-018-8779-x. Epub 2018 Mar 6. J Neurol. 2018. PMID: 29511863
-
Multifaceted Biomarkers Suggest a Similar Profile of CNS Pathology in Relapsing and Progressive MS.Eur J Neurol. 2025 Feb;32(2):e70052. doi: 10.1111/ene.70052. Eur J Neurol. 2025. PMID: 39907163 Free PMC article.
-
The MRZ reaction as a highly specific marker of multiple sclerosis: re-evaluation and structured review of the literature.J Neurol. 2017 Mar;264(3):453-466. doi: 10.1007/s00415-016-8360-4. Epub 2016 Dec 22. J Neurol. 2017. PMID: 28005176 Review.
-
Neuronal and glial CSF biomarkers in multiple sclerosis: a systematic review and meta-analysis.Rev Neurosci. 2021 Feb 17;32(6):573-595. doi: 10.1515/revneuro-2020-0145. Print 2021 Aug 26. Rev Neurosci. 2021. PMID: 33594840
Cited by
-
Evodiae Fructus extract suppresses inflammatory response in HaCaT cells and improves house dust mite-induced atopic dermatitis in NC/Nga mice.Sci Rep. 2024 Jan 4;14(1):472. doi: 10.1038/s41598-023-50257-3. Sci Rep. 2024. PMID: 38172219 Free PMC article.
-
Intrathecal immune reactivity against Measles-, Rubella-, and Varicella Zoster viruses is associated with cerebrospinal fluid inflammation in multiple sclerosis.Mult Scler. 2024 Nov;30(13):1598-1608. doi: 10.1177/13524585241279645. Epub 2024 Oct 8. Mult Scler. 2024. PMID: 39377663 Free PMC article.
-
Serum glial fibrillary acidic protein and disability progression in progressive multiple sclerosis.Ann Clin Transl Neurol. 2024 Feb;11(2):477-485. doi: 10.1002/acn3.51969. Epub 2023 Dec 19. Ann Clin Transl Neurol. 2024. PMID: 38111972 Free PMC article.
-
The multifaceted anticancer potential of luteolin: involvement of NF-κB, AMPK/mTOR, PI3K/Akt, MAPK, and Wnt/β-catenin pathways.Inflammopharmacology. 2025 Feb;33(2):505-525. doi: 10.1007/s10787-024-01596-8. Epub 2024 Nov 14. Inflammopharmacology. 2025. PMID: 39543054 Review.
-
The Presence of MRZ Reactions Improves the Prediction of Multiple Sclerosis in Children with Optic Neuritis.Children (Basel). 2025 Apr 13;12(4):497. doi: 10.3390/children12040497. Children (Basel). 2025. PMID: 40310118 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

