Monitoring Endothelin-A Receptor Expression during the Progression of Atherosclerosis
- PMID: 33255872
- PMCID: PMC7761144
- DOI: 10.3390/biomedicines8120538
Monitoring Endothelin-A Receptor Expression during the Progression of Atherosclerosis
Abstract
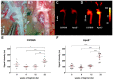
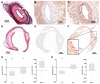
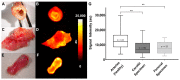
Cardiovascular disease remains the most frequent cause of death worldwide. Atherosclerosis, an underlying cause of cardiovascular disease, is an inflammatory disorder associated with endothelial dysfunction. The endothelin system plays a crucial role in the pathogenesis of endothelial dysfunction and is involved in the development of atherosclerosis. We aimed to reveal the expression levels of the endothelin-A receptor (ETAR) in the course of atherogenesis to reveal possible time frames for targeted imaging and interventions. We used the ApoE-/- mice model and human specimens and evaluated ETAR expression by quantitative rtPCR (qPCR), histology and fluorescence molecular imaging. We found a significant upregulation of ETAR after 22 weeks of high-fat diet in the aortae of ApoE-/- mice. With regard to translation to human disease, we applied the fluorescent probe to fresh explants of human carotid and femoral artery specimens. The findings were correlated with qPCR and histology. While ETAR is upregulated during the progression of early atherosclerosis in the ApoE-/- mouse model, we found that ETAR expression is substantially reduced in advanced human atherosclerotic plaques. Moreover, those expression changes were clearly depicted by fluorescence imaging using our in-house designed ETAR-Cy 5.5 probe confirming its specificity and potential use in future studies.
Keywords: ApoE-KnockOut; atherosclerosis; carotid endarterectomy; endothelin receptor expression; endothelin system; fluorescence imaging; molecular imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

