Differential Timing and Coordination of Neurogenesis and Astrogenesis in Developing Mouse Hippocampal Subregions
- PMID: 33255945
- PMCID: PMC7760658
- DOI: 10.3390/brainsci10120909
Differential Timing and Coordination of Neurogenesis and Astrogenesis in Developing Mouse Hippocampal Subregions
Abstract
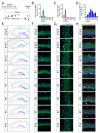
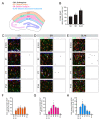
Neocortical development has been extensively studied and therefore is the basis of our understanding of mammalian brain development. One fundamental principle of neocortical development is that neurogenesis and gliogenesis are temporally segregated processes. However, it is unclear how neurogenesis and gliogenesis are coordinated in non-neocortical regions of the cerebral cortex, such as the hippocampus, also known as the archicortex. Here, we show that the timing of neurogenesis and astrogenesis in the Cornu Ammonis (CA) 1 and CA3 regions of mouse hippocampus mirrors that of the neocortex; neurogenesis occurs embryonically, followed by astrogenesis during early postnatal development. In contrast, we find that neurogenesis in the dentate gyrus begins embryonically but is a protracted process which peaks neonatally and continues at low levels postnatally. As a result, astrogenesis, which occurs during early postnatal development, overlaps with the process of neurogenesis in the dentate gyrus. During all stages, neurogenesis overwhelms astrogenesis in the dentate gyrus. In addition, we find that the timing of peak astrogenesis varies by hippocampal subregion. Together, our results show differential timing and coordination of neurogenesis and astrogenesis in developing mouse hippocampal subregions and suggest that neurogenesis and gliogenesis occur simultaneously during dentate gyrus development, challenging the conventional principle that neurogenesis and gliogenesis are temporally separated processes.
Keywords: astrogenesis; birth-dating; cytogenesis; hippocampus; neurogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

