Muscle Stem Cell-Derived Extracellular Vesicles Reverse Hydrogen Peroxide-Induced Mitochondrial Dysfunction in Mouse Myotubes
- PMID: 33256005
- PMCID: PMC7760380
- DOI: 10.3390/cells9122544
Muscle Stem Cell-Derived Extracellular Vesicles Reverse Hydrogen Peroxide-Induced Mitochondrial Dysfunction in Mouse Myotubes
Abstract
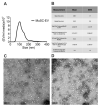
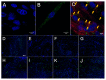
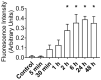
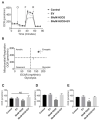
Muscle stem cells (MuSCs) hold great potential as a regenerative therapeutic but have met numerous challenges in treating systemic muscle diseases. Muscle stem cell-derived extracellular vesicles (MuSC-EVs) may overcome these limitations. We assessed the number and size distribution of extracellular vesicles (EVs) released by MuSCs ex vivo, determined the extent to which MuSC-EVs deliver molecular cargo to myotubes in vitro, and quantified MuSC-EV-mediated restoration of mitochondrial function following oxidative injury. MuSCs released an abundance of EVs in culture. MuSC-EVs delivered protein cargo into myotubes within 2 h of incubation. Fluorescent labeling of intracellular mitochondria showed co-localization of delivered protein and mitochondria. Oxidatively injured myotubes demonstrated a significant decline in maximal oxygen consumption rate and spare respiratory capacity relative to untreated myotubes. Remarkably, subsequent treatment with MuSC-EVs significantly improved maximal oxygen consumption rate and spare respiratory capacity relative to the myotubes that were damaged but received no subsequent treatment. Surprisingly, MuSC-EVs did not affect mitochondrial function in undamaged myotubes, suggesting the cargo delivered is able to repair but does not expand the existing mitochondrial network. These data demonstrate that MuSC-EVs rapidly deliver proteins into myotubes, a portion of which co-localizes with mitochondria, and reverses mitochondria dysfunction in oxidatively-damaged myotubes.
Keywords: cachexia; extracellular vesicles; mitochondria; muscle stem cells; muscular dystrophy; oxidative stress; skeletal muscle.
Conflict of interest statement
M.B.H. and J.T.S. are founders and majority equity holders of extracellular vesicle-based company Extrave Bioscience, L.L.C. Extrave Bioscience, L.L.C. has licensed intellectual property from the University of Delaware related to some of the findings presented in the present study.
Figures

Similar articles
-
Extracellular Vesicles Released From Skeletal Muscle Post-Chronic Contractile Activity Increase Mitochondrial Biogenesis in Recipient Myoblasts.J Extracell Vesicles. 2025 Apr;14(4):e70045. doi: 10.1002/jev2.70045. J Extracell Vesicles. 2025. PMID: 40205946 Free PMC article.
-
Extracellular Vesicles Released by Oxidatively Injured or Intact C2C12 Myotubes Promote Distinct Responses Converging toward Myogenesis.Int J Mol Sci. 2017 Nov 22;18(11):2488. doi: 10.3390/ijms18112488. Int J Mol Sci. 2017. PMID: 29165341 Free PMC article.
-
Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes.Free Radic Biol Med. 2012 Jan 1;52(1):198-207. doi: 10.1016/j.freeradbiomed.2011.10.449. Epub 2011 Oct 25. Free Radic Biol Med. 2012. PMID: 22080086 Free PMC article.
-
Role of extracellular vesicles in mitochondrial eye diseases.IUBMB Life. 2022 Dec;74(12):1264-1272. doi: 10.1002/iub.2687. Epub 2022 Nov 11. IUBMB Life. 2022. PMID: 36308309 Free PMC article. Review.
-
Delivery of mitochondria-containing extracellular vesicles to the BBB for ischemic stroke therapy.Expert Opin Drug Deliv. 2023 Jul-Dec;20(12):1769-1788. doi: 10.1080/17425247.2023.2279115. Epub 2023 Dec 29. Expert Opin Drug Deliv. 2023. PMID: 37921194 Review.
Cited by
-
The Double-Edged Role of Extracellular Vesicles in the Hallmarks of Aging.Biomolecules. 2023 Jan 13;13(1):165. doi: 10.3390/biom13010165. Biomolecules. 2023. PMID: 36671550 Free PMC article. Review.
-
Extracellular Vesicles Released From Skeletal Muscle Post-Chronic Contractile Activity Increase Mitochondrial Biogenesis in Recipient Myoblasts.J Extracell Vesicles. 2025 Apr;14(4):e70045. doi: 10.1002/jev2.70045. J Extracell Vesicles. 2025. PMID: 40205946 Free PMC article.
-
Defining the influence of size-exclusion chromatography fraction window and ultrafiltration column choice on extracellular vesicle recovery in a skeletal muscle model.J Extracell Biol. 2023 Apr 21;2(4):e85. doi: 10.1002/jex2.85. eCollection 2023 Apr. J Extracell Biol. 2023. PMID: 38939692 Free PMC article.
-
The extracellular vesicle proteomes of Sorghum bicolor and Arabidopsis thaliana are partially conserved.Plant Physiol. 2024 Feb 29;194(3):1481-1497. doi: 10.1093/plphys/kiad644. Plant Physiol. 2024. PMID: 38048422 Free PMC article.
-
Interactions of mitochondrial and skeletal muscle biology in mitochondrial myopathy.Biochem J. 2023 Nov 15;480(21):1767-1789. doi: 10.1042/BCJ20220233. Biochem J. 2023. PMID: 37965929 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

