Valproic Acid and Its Amidic Derivatives as New Antivirals against Alphaherpesviruses
- PMID: 33256172
- PMCID: PMC7760627
- DOI: 10.3390/v12121356
Valproic Acid and Its Amidic Derivatives as New Antivirals against Alphaherpesviruses
Abstract
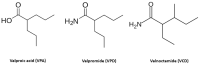
Herpes simplex viruses (HSVs) are neurotropic viruses with broad host range whose infections cause considerable health problems in both animals and humans. In fact, 67% of the global population under the age of 50 are infected with HSV-1 and 13% have clinically recurrent HSV-2 infections. The most prescribed antiherpetics are nucleoside analogues such as acyclovir, but the emergence of mutants resistant to these drugs and the lack of available vaccines against human HSVs has led to an imminent need for new antivirals. Valproic acid (VPA) is a branched short-chain fatty acid clinically used as a broad-spectrum antiepileptic drug in the treatment of neurological disorders, which has shown promising antiviral activity against some herpesviruses. Moreover, its amidic derivatives valpromide and valnoctamide also share this antiherpetic activity. This review summarizes the current research on the use of VPA and its amidic derivatives as alternatives to traditional antiherpetics in the fight against HSV infections.
Keywords: alphaherpesvirus; valnoctamide; valproic acid; valpromide.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Amidic derivatives of valproic acid, valpromide and valnoctamide, inhibit HSV-1 infection in oligodendrocytes.Antiviral Res. 2019 Aug;168:91-99. doi: 10.1016/j.antiviral.2019.05.006. Epub 2019 May 25. Antiviral Res. 2019. PMID: 31132386
-
Amidic modification of valproic acid reduces skeletal teratogenicity in mice.Birth Defects Res B Dev Reprod Toxicol. 2004 Feb;71(1):47-53. doi: 10.1002/bdrb.10057. Birth Defects Res B Dev Reprod Toxicol. 2004. PMID: 14991910
-
Structure-pharmacokinetic relationships in a series of valpromide derivatives with antiepileptic activity.Pharm Res. 1989 Aug;6(8):683-9. doi: 10.1023/a:1015934321764. Pharm Res. 1989. PMID: 2510141
-
New CNS-active drugs which are second-generation valproic acid: can they lead to the development of a magic bullet?Curr Opin Neurol. 2003 Apr;16(2):203-11. doi: 10.1097/01.wco.0000063774.81810.30. Curr Opin Neurol. 2003. PMID: 12644750 Review.
-
Insights into Structural Modifications of Valproic Acid and Their Pharmacological Profile.Molecules. 2021 Dec 24;27(1):104. doi: 10.3390/molecules27010104. Molecules. 2021. PMID: 35011339 Free PMC article. Review.
Cited by
-
Gene crosstalk between COVID-19 and preeclampsia revealed by blood transcriptome analysis.Front Immunol. 2024 Jan 8;14:1243450. doi: 10.3389/fimmu.2023.1243450. eCollection 2023. Front Immunol. 2024. PMID: 38259479 Free PMC article.
-
Nutraceutical Curcumin with Promising Protection against Herpesvirus Infections and Their Associated Inflammation: Mechanisms and Pathways.Microorganisms. 2021 Jan 31;9(2):292. doi: 10.3390/microorganisms9020292. Microorganisms. 2021. PMID: 33572685 Free PMC article. Review.
-
Pseudorabies virus uses clathrin mediated endocytosis to enter PK15 swine cell line.Front Microbiol. 2024 Feb 5;15:1332175. doi: 10.3389/fmicb.2024.1332175. eCollection 2024. Front Microbiol. 2024. PMID: 38374920 Free PMC article.
-
[Valproic Acid Could Help in the Fight Against COVID-19: a case-control study].Neurologia. 2022 Feb 14. doi: 10.1016/j.nrl.2022.01.007. Online ahead of print. Neurologia. 2022. PMID: 35185237 Free PMC article.
-
Drug repositioning as a promising approach for the eradication of emerging and re-emerging viral agents.Mol Divers. 2025 Mar 18. doi: 10.1007/s11030-025-11131-8. Online ahead of print. Mol Divers. 2025. PMID: 40100484 Review.
References
-
- Carrasco L., Almendral J.M. Virus Patógenos. Editorial Hélice, Fundación BBVA; Madrid, Spain: 2006.
-
- Smith T.T., Whitley R.J. Infectious Diseases. Elsevier; Amsterdam, The Netherlands: 2017. Herpesviruses; pp. 1426–1438.e1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources