Gill Mucus and Gill Mucin O-glycosylation in Healthy and Amebic Gill Disease-Affected Atlantic Salmon
- PMID: 33256221
- PMCID: PMC7768351
- DOI: 10.3390/microorganisms8121871
Gill Mucus and Gill Mucin O-glycosylation in Healthy and Amebic Gill Disease-Affected Atlantic Salmon
Abstract
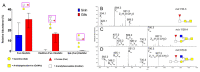
Amoebic gill disease (AGD) causes poor performance and death in salmonids. Mucins are mainly comprised by carbohydrates and are main components of the mucus covering the gill. Since glycans regulate pathogen binding and growth, glycosylation changes may affect susceptibility to primary and secondary infections. We investigated gill mucin O-glycosylation from Atlantic salmon with and without AGD using liquid chromatography-mass spectrometry. Gill mucin glycans were larger and more complex, diverse and fucosylated than skin mucins. Confocal microscopy revealed that fucosylated mucus coated sialylated mucus strands in ex vivo gill mucus. Terminal HexNAcs were more abundant among O-glycans from AGD-affected Atlantic salmon, whereas core 1 structures and structures with acidic moieties such as N-acetylneuraminic acid (NeuAc) and sulfate groups were less abundant compared to non-infected fish. The fucosylated and NeuAc-containing O-glycans were inversely proportional, with infected fish on the lower scale of NeuAc abundance and high on fucosylated structures. The fucosylated epitopes were of three types: Fuc-HexNAc-R, Gal-[Fuc-]HexNAc-R and HexNAc-[Fuc-]HexNAc-R. These blood group-like structures could be an avenue to diversify the glycan repertoire to limit infection in the exposed gills. Furthermore, care must be taken when using skin mucus as proxy for gill mucus, as gill mucins are distinctly different from skin mucins.
Keywords: Atlantic salmon; Neoparamoeba perurans; amebic gill disease; gill; glycosylation; mucin; mucosal immunology; mucus; parasite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Benktander J., Venkatakrishnan V., Padra J.T., Sundh H., Sundell K., Murughan A.V.M., Maynard B., Linden S.K. Effects of size and geographical origin on Atlantic salmon, Salmo salar, mucin O-glycan repertoire. Mol. Cell. Proteom. 2019;18:1183–1196. doi: 10.1074/mcp.RA119.001319. - DOI - PMC - PubMed
-
- Padra M., Adamczyk B., Flahou B., Erhardsson M., Chahal G., Smet A., Jin C., Thorell A., Ducatelle R., Haesebrouck F., et al. Helicobacter suis infection alters glycosylation and decreases the pathogen growth inhibiting effect and binding avidity of gastric mucins. Mucosal Immunol. 2019;12:784–794. doi: 10.1038/s41385-019-0154-4. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

