The Function of Transthyretin Complexes with Metallothionein in Alzheimer's Disease
- PMID: 33256250
- PMCID: PMC7730073
- DOI: 10.3390/ijms21239003
The Function of Transthyretin Complexes with Metallothionein in Alzheimer's Disease
Abstract
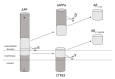
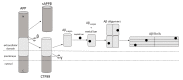
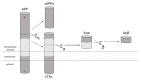
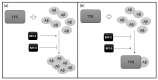
Alzheimer's disease (AD) is one of the most frequently diagnosed types of dementia in the elderly. An important pathological feature in AD is the aggregation and deposition of the β-amyloid (Aβ) in extracellular plaques. Transthyretin (TTR) can cleave Aβ, resulting in the formation of short peptides with less activity of amyloid plaques formation, as well as being able to degrade Aβ peptides that have already been aggregated. In the presence of TTR, Aβ aggregation decreases and toxicity of Aβ is abolished. This may prevent amyloidosis but the malfunction of this process leads to the development of AD. In the context of Aβplaque formation in AD, we discuss metallothionein (MT) interaction with TTR, the effects of which depend on the type of MT isoform. In the brains of patients with AD, the loss of MT-3 occurs. On the contrary, MT-1/2 level has been consistently reported to be increased. Through interaction with TTR, MT-2 reduces the ability of TTR to bind to Aβ, while MT-3 causes the opposite effect. It increases TTR-Aβ binding, providing inhibition of Aβ aggregation. The protective effect, assigned to MT-3 against the deposition of Aβ, relies also on this mechanism. Additionally, both Zn7MT-2 and Zn7MT-3, decrease Aβ neurotoxicity in cultured cortical neurons probably because of a metal swap between Zn7MT and Cu(II)Aβ. Understanding the molecular mechanism of metals transfer between MT and other proteins as well as cognition of the significance of TTR interaction with different MT isoforms can help in AD treatment and prevention.
Keywords: Alzheimer’s disease; metallothionein; protein-protein interaction; transthyretin; β-amyloid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The native copper- and zinc-binding protein metallothionein blocks copper-mediated Abeta aggregation and toxicity in rat cortical neurons.PLoS One. 2010 Aug 11;5(8):e12030. doi: 10.1371/journal.pone.0012030. PLoS One. 2010. PMID: 20711450 Free PMC article.
-
Transthyretin stabilization by iododiflunisal promotes amyloid-β peptide clearance, decreases its deposition, and ameliorates cognitive deficits in an Alzheimer's disease mouse model.J Alzheimers Dis. 2014;39(2):357-70. doi: 10.3233/JAD-131355. J Alzheimers Dis. 2014. PMID: 24169237
-
Neuronal production of transthyretin in human and murine Alzheimer's disease: is it protective?J Neurosci. 2011 Aug 31;31(35):12483-90. doi: 10.1523/JNEUROSCI.2417-11.2011. J Neurosci. 2011. PMID: 21880910 Free PMC article.
-
Tetrameric Transthyretin as a Protective Factor Against Alzheimer's Disease.Mol Neurobiol. 2025 Mar;62(3):2945-2954. doi: 10.1007/s12035-024-04442-8. Epub 2024 Aug 27. Mol Neurobiol. 2025. PMID: 39192044 Free PMC article. Review.
-
The Positive Side of the Alzheimer's Disease Amyloid Cross-Interactions: The Case of the Aβ 1-42 Peptide with Tau, TTR, CysC, and ApoA1.Molecules. 2020 May 23;25(10):2439. doi: 10.3390/molecules25102439. Molecules. 2020. PMID: 32456156 Free PMC article. Review.
Cited by
-
Metallothionein: A Comprehensive Review of Its Classification, Structure, Biological Functions, and Applications.Antioxidants (Basel). 2024 Jul 9;13(7):825. doi: 10.3390/antiox13070825. Antioxidants (Basel). 2024. PMID: 39061894 Free PMC article. Review.
-
Unravelling the myriad physiologic roles of transthyretin: critical considerations for treating transthyretin amyloidosis.Ann Med. 2025 Dec;57(1):2536755. doi: 10.1080/07853890.2025.2536755. Epub 2025 Jul 27. Ann Med. 2025. PMID: 40717228 Free PMC article. Review.
-
Modeling the early stages of Alzheimer's disease by administering intracerebroventricular injections of human native Aβ oligomers to rats.Acta Neuropathol Commun. 2022 Aug 16;10(1):113. doi: 10.1186/s40478-022-01417-5. Acta Neuropathol Commun. 2022. PMID: 35974377 Free PMC article.
-
Transthyretin as a therapeutic target: the future of disease-modifying therapies for Alzheimer's disease.Mol Biol Rep. 2025 Apr 8;52(1):370. doi: 10.1007/s11033-025-10485-4. Mol Biol Rep. 2025. PMID: 40195175 Review.
-
Cellular zinc metabolism and zinc signaling: from biological functions to diseases and therapeutic targets.Signal Transduct Target Ther. 2024 Jan 3;9(1):6. doi: 10.1038/s41392-023-01679-y. Signal Transduct Target Ther. 2024. PMID: 38169461 Free PMC article. Review.
References
-
- Hyman B.T., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Carrillo M.C., Dickson D.W., Duyckaerts C., Frosch M.P., Masliah E., et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

