Treatment of patients with MYC rearrangement positive large B-cell lymphoma with R-CHOP plus lenalidomide: results of a multicenter HOVON phase II trial
- PMID: 33256379
- PMCID: PMC7716355
- DOI: 10.3324/haematol.2019.238162
Treatment of patients with MYC rearrangement positive large B-cell lymphoma with R-CHOP plus lenalidomide: results of a multicenter HOVON phase II trial
Abstract
Patients with MYC-rearrangement positive large B-cell lymphoma (MYC+ LBCL) have an inferior prognosis following standard first-line therapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP) as compared to patients without MYC rearrangement. Although intensive chemotherapy regimens yield higher remission rates, toxicity remains a concern. Lenalidomide is an oral immunomodulatory drug which downregulates MYC and its target genes thereby providing support using lenalidomide as additional therapeutic option for MYC+ LBCL. A phase II trial was conducted evaluating the efficacy of lenalidomide (15 mg day 1-14) in combination with R-CHOP (R2CHOP) in newly diagnosed MYC+ LBCL patients identified through a nationwide MYC-FISH screening program. The primary endpoint was complete metabolic response (CMR) on centrally reviewed 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)-computer tomography (CT)-scan at end-of-treatment. Secondary endpoints were overall survival (OS), disease-free survival (DFS) and event-free survival (EFS). Eighty-two patients with stage II-IV MYC+ LBCL were treated with 6 cycles of R2CHOP. At EOT, 67% (confidence interval (CI) 58-75%) of the patients reached CMR. With a median follow-up of 25.4 months, 2-year estimates (95% CI) for OS, DFS, EFS were 73% (62-82%), 75% (63-84%) and 63% (52-73%) respectively. In this prospective trial for newly diagnosed MYC+ LBCL patients, we found that administering R2CHOP was safe, and yields comparable CMR and survival rates as in studies applying more intensive chemotherapy regimens. Hence, these findings offer new prospects for MYC+ LBCL patients and warrant comparison in prospective randomized clinical trials. This trial was registered at www.clinicaltrialsregister.eu (#2014-002654-39).
Figures
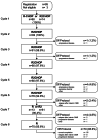

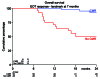
References
-
- Ziepert M, Hasenclever D, Kuhnt E, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(14):2373-2380. - PubMed
-
- Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166(6):891-901. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

