BMP Ligand Trap ALK3-Fc Attenuates Osteogenesis and Heterotopic Ossification in Blast-Related Lower Extremity Trauma
- PMID: 33256557
- PMCID: PMC7826435
- DOI: 10.1089/scd.2020.0162
BMP Ligand Trap ALK3-Fc Attenuates Osteogenesis and Heterotopic Ossification in Blast-Related Lower Extremity Trauma
Abstract
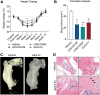
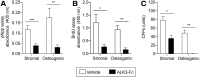
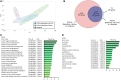
Traumatic heterotopic ossification (tHO) commonly develops in wounded service members who sustain high-energy and blast-related traumatic amputations. Currently, no safe and effective preventive measures have been identified for this patient population. Bone morphogenetic protein (BMP) signaling blockade has previously been shown to reduce ectopic bone formation in genetic models of HO. In this study, we demonstrate the efficacy of small-molecule inhibition with LDN193189 (ALK2/ALK3 inhibition), LDN212854 (ALK2-biased inhibition), and BMP ligand trap ALK3-Fc at inhibiting early and late osteogenic differentiation of tissue-resident mesenchymal progenitor cells (MPCs) harvested from mice subjected to burn/tenotomy, a well-characterized trauma-induced model of HO. Using an established rat tHO model of blast-related extremity trauma and methicillin-resistant Staphylococcus aureus infection, a significant decrease in ectopic bone volume was observed by micro-computed tomography imaging following treatment with LDN193189, LDN212854, and ALK3-Fc. The efficacy of LDN193189 and LDN212854 in this model was associated with weight loss (17%-19%) within the first two postoperative weeks, and in the case of LDN193189, delayed wound healing and metastatic infection was observed, while ALK3-Fc was well tolerated. At day 14 following injury, RNA-Seq and quantitative reverse transcriptase-polymerase chain reaction analysis revealed that ALK3-Fc enhanced the expression of skeletal muscle structural genes and myogenic transcriptional factors while inhibiting the expression of inflammatory genes. Tissue-resident MPCs harvested from rats treated with ALK3-Fc exhibited reduced osteogenic differentiation, proliferation, and self-renewal capacity and diminished expression of genes associated with endochondral ossification and SMAD-dependent signaling pathways. Together, these results confirm the contribution of BMP signaling in osteogenic differentiation and ectopic bone formation and that a selective ligand-trap approach such as ALK3-Fc may be an effective and tolerable prophylactic strategy for tHO.
Keywords: ALK2/ALK3 kinase inhibitors; BMPRIA; bone morphogenic protein (BMP); heterotopic ossification; limb amputation; trauma-induced extremity injury.
Conflict of interest statement
No competing financial interests exist.
Figures
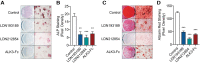


Similar articles
-
Small molecule inhibition of non-canonical (TAK1-mediated) BMP signaling results in reduced chondrogenic ossification and heterotopic ossification in a rat model of blast-associated combat-related lower limb trauma.Bone. 2020 Oct;139:115517. doi: 10.1016/j.bone.2020.115517. Epub 2020 Jul 2. Bone. 2020. PMID: 32622875 Free PMC article.
-
Targeted stimulation of retinoic acid receptor-γ mitigates the formation of heterotopic ossification in an established blast-related traumatic injury model.Bone. 2016 Sep;90:159-67. doi: 10.1016/j.bone.2016.06.014. Epub 2016 Jun 28. Bone. 2016. PMID: 27368930 Free PMC article.
-
High Frequency Spectral Ultrasound Imaging Detects Early Heterotopic Ossification in Rodents.Stem Cells Dev. 2021 May 1;30(9):473-484. doi: 10.1089/scd.2021.0011. Epub 2021 Apr 19. Stem Cells Dev. 2021. PMID: 33715398 Free PMC article.
-
BMP and mTOR signaling in heterotopic ossification: Does their crosstalk provide therapeutic opportunities?J Cell Biochem. 2019 Aug;120(8):12108-12122. doi: 10.1002/jcb.28710. Epub 2019 Apr 15. J Cell Biochem. 2019. PMID: 30989716 Review.
-
A review of the biomarkers and in vivo models for the diagnosis and treatment of heterotopic ossification following blast and trauma-induced injuries.Bone. 2021 Feb;143:115765. doi: 10.1016/j.bone.2020.115765. Epub 2020 Dec 4. Bone. 2021. PMID: 33285256 Review.
Cited by
-
Pathophysiology and Emerging Molecular Therapeutic Targets in Heterotopic Ossification.Int J Mol Sci. 2022 Jun 23;23(13):6983. doi: 10.3390/ijms23136983. Int J Mol Sci. 2022. PMID: 35805978 Free PMC article. Review.
-
Heterotopic ossification: Current developments and emerging potential therapies.Chin Med J (Engl). 2025 Feb 20;138(4):389-404. doi: 10.1097/CM9.0000000000003244. Epub 2025 Jan 17. Chin Med J (Engl). 2025. PMID: 39819765 Free PMC article. Review.
-
Intersections of Fibrodysplasia Ossificans Progressiva and Traumatic Heterotopic Ossification.Biomolecules. 2024 Mar 14;14(3):349. doi: 10.3390/biom14030349. Biomolecules. 2024. PMID: 38540768 Free PMC article. Review.
-
Activin receptor-like kinase 3: a critical modulator of development and function of mineralized tissues.Front Cell Dev Biol. 2023 Jun 30;11:1209817. doi: 10.3389/fcell.2023.1209817. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37457289 Free PMC article. Review.
-
Functionally diverse heteromeric traps for ligands of the transforming growth factor-β superfamily.Sci Rep. 2021 Sep 15;11(1):18341. doi: 10.1038/s41598-021-97203-9. Sci Rep. 2021. PMID: 34526551 Free PMC article.
References
-
- Potter BK, Burns TC, Lacap AP, Granville RR and Gajewski D (2006). Heterotopic ossification in the residual limbs of traumatic and combat-related amputees. J Am Acad Orthop Surg 14:S191–S197 - PubMed
-
- Potter BK, Burns TC, Lacap AP, Granville RR and Gajewski DA (2007). Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am 89:476–486 - PubMed
-
- Melcer T, Belnap B, Walker GJ, Konoske P and Galarneau M (2011). Heterotopic ossification in combat amputees from Afghanistan and Iraq wars: five case histories and results from a small series of patients. J Rehabil Res Dev 48:1–12 - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and Marshak DR (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

