Effects of a movement control and tactile acuity training in patients with nonspecific chronic low back pain and control impairment - a randomised controlled pilot study
- PMID: 33256694
- PMCID: PMC7702711
- DOI: 10.1186/s12891-020-03727-y
Effects of a movement control and tactile acuity training in patients with nonspecific chronic low back pain and control impairment - a randomised controlled pilot study
Abstract
Background: Nonspecific chronic low back pain (NSCLBP) is a heterogeneous condition that is associated with complex neuromuscular adaptations. Exercise is a widely administered treatment, but its effects are small to moderate. Tailoring patient-specific exercise treatments based on subgroup classification may improve patient outcomes.
Objective: In this randomised controlled pilot study, our objective was to compare the feasibility and possible effects of a specific sensorimotor treatment (SMT) with those of a general exercise (GE) programme on patients with NSCLBP and control impairment (CI).
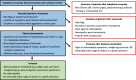
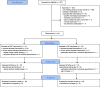
Methods: Patients with NSCLBP and CI were randomised into an SMT or a GE programme spanning 6 sessions each. The feasibility criteria included the study design, assessments, interventions and magnitudes of effects, and costs. Adverse events were documented. Primary (pain, physical function, and quality of life) and secondary outcomes were assessed three times: twice at baseline (t1a and t1b) to estimate parameter stability and once after the intervention (t2).
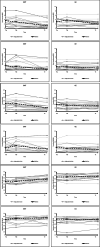
Results: Two-hundred and twenty-seven patients were screened to include 34 participants with NSCLBP and CI. Both treatment programmes and the assessments seemed feasible because their durations and contents were perceived as adequate. The total cost per participant was €321. Two adverse events occurred (one not likely related to the SMT, one likely related to the GE intervention). The SMT showed a tendency for superior effects in terms of pain severity (SMT t1a 3.5, t2 1.1; GE t1a 3.0, t2 2.0), pain interference (SMT t1a 1.9, t2 0.4; GE t1a 1.5, t2 0.9), physical component of quality of life (SMT t1a 39, t2 46; GE t1a 45, t2 48), and movement control.
Conclusions: The SMT approach proposed in this study is feasible and should be tested thoroughly in future studies, possibly as an addition to GE. To ensure the detection of differences in pain severity between SMT and GE in patients with NSCLBP with 80% power, future studies should include 110 patients. If the current results are confirmed, SMT should be considered in interventions for patients with NSCLBP and CI.
Trial registration: Registered in the German Register for Clinical Trials (Trial registration date: November 11, 2016; Trial registration number: DRKS00011063 ; URL of trial registry record); retrospectively registered.
Keywords: Chronic low back pain; Feasibility; Low back pain; Motor control; Physical therapy modalities; Tactile acuity.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources