Logistics of an advanced therapy medicinal product during COVID-19 pandemic in Italy: successful delivery of mesenchymal stromal cells in dry ice
- PMID: 33256746
- PMCID: PMC7702210
- DOI: 10.1186/s12967-020-02625-0
Logistics of an advanced therapy medicinal product during COVID-19 pandemic in Italy: successful delivery of mesenchymal stromal cells in dry ice
Abstract
Background: During the coronavirus disease-2019 (COVID-19) pandemic, Italian hospitals faced the most daunting challenges of their recent history, and only essential therapeutic interventions were feasible. From March to April 2020, the Laboratory of Advanced Cellular Therapies (Vicenza, Italy) received requests to treat a patient with severe COVID-19 and a patient with acute graft-versus-host disease with umbilical cord-derived mesenchymal stromal cells (UC-MSCs). Access to clinics was restricted due to the risk of contagion. Transport of UC-MSCs in liquid nitrogen was unmanageable, leaving shipment in dry ice as the only option.
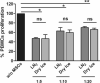
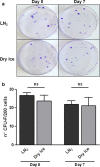
Methods: We assessed effects of the transition from liquid nitrogen to dry ice on cell viability; apoptosis; phenotype; proliferation; immunomodulation; and clonogenesis; and validated dry ice-based transport of UC-MSCs to clinics.
Results: Our results showed no differences in cell functionality related to the two storage conditions, and demonstrated the preservation of immunomodulatory and clonogenic potentials in dry ice. UC-MSCs were successfully delivered to points-of-care, enabling favourable clinical outcomes.
Conclusions: This experience underscores the flexibility of a public cell factory in its adaptation of the logistics of an advanced therapy medicinal product during a public health crisis. Alternative supply chains should be evaluated for other cell products to guarantee delivery during catastrophes.
Keywords: COVID-19; GvHD; Mesenchymal stromal cells; Supply chain.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Mesenchymal Stem Cell-Based Therapy for COVID-19: Possibility and Potential.Curr Stem Cell Res Ther. 2021;16(2):105-108. doi: 10.2174/1574888X15666200601152832. Curr Stem Cell Res Ther. 2021. PMID: 32479246 Review.
-
Systematic review and meta-analysis of cell therapy for COVID-19: global clinical trial landscape, published safety/efficacy outcomes, cell product manufacturing and clinical delivery.Front Immunol. 2023 Jun 21;14:1200180. doi: 10.3389/fimmu.2023.1200180. eCollection 2023. Front Immunol. 2023. PMID: 37415976 Free PMC article.
-
Comparison of Mesenchymal Stromal Cells From Different Origins for the Treatment of Graft-vs.-Host-Disease in a Humanized Mouse Model.Front Immunol. 2019 Apr 2;10:619. doi: 10.3389/fimmu.2019.00619. eCollection 2019. Front Immunol. 2019. PMID: 31001253 Free PMC article.
-
Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial.Stem Cells Transl Med. 2021 Sep;10(9):1279-1287. doi: 10.1002/sctm.21-0046. Epub 2021 Jun 8. Stem Cells Transl Med. 2021. PMID: 34102020 Free PMC article. Clinical Trial.
-
Human Umbilical Cord Mesenchymal Stromal Cell Treatment of Severe COVID-19 Patients: A 3-Month Follow-Up Study Following Hospital Discharge.Stem Cells Dev. 2021 Aug 1;30(15):773-781. doi: 10.1089/scd.2021.0015. Epub 2021 Jun 29. Stem Cells Dev. 2021. PMID: 34044609
References
-
- Massie I, Selden C, Hodgson H, Fuller B. Storage temperatures for cold-chain delivery in cell therapy: a study of alginate-encapsulated liver cell spheroids stored at − 80 degrees c or − 170 degrees c for up to 1 year. Tissue Eng Part C Methods. 2013;19(3):189–195. doi: 10.1089/ten.tec.2012.0307. - DOI - PMC - PubMed
-
- Amati E, Perbellini O, Rotta G, Bernardi M, Chieregato K, Sella S, et al. High-throughput immunophenotypic characterization of bone marrow- and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources. Stem Cell Res Therapy. 2018;9:10. doi: 10.1186/s13287-017-0755-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

