Diagnostic journey and impact of enzyme replacement therapy for mucopolysaccharidosis IVA: a sibling control study
- PMID: 33256811
- PMCID: PMC7706253
- DOI: 10.1186/s13023-020-01618-y
Diagnostic journey and impact of enzyme replacement therapy for mucopolysaccharidosis IVA: a sibling control study
Abstract
Background: Mucopolysaccharidosis (MPS) IVA, also known as Morquio A syndrome, is a rare autosomal recessive lysosomal storage disorder caused by a deficiency in the enzyme N-acetylgalactosamine-6-sulfatase. Early recognition, diagnosis, and treatment of this progressive, multisystem disease by enzyme replacement therapy (ERT) can lead to improved outcomes and reduced mortality.
Methods: This report documents the diagnostic journey and treatment with ERT of three siblings with MPS IVA. Clinical outcome measures included growth, endurance, imaging, cardiac, respiratory, ophthalmology, and laboratory evaluations.
Results: Three siblings, diagnosed at 14.7, 10.1, and 3.2 years of age, demonstrated clinical improvement with weekly infusions of 2.0 mg/kg elosulfase alfa (Vimizim®, BioMarin Pharmaceutical, Novato, CA, USA). Patient 1 (oldest sibling) and Patient 2 (middle sibling) experienced a diagnostic delay of 8 years 7 months and 4 years after symptom onset, respectively. All three patients demonstrated improvements in growth, 6-min walk distance, joint range of motion, and respiratory function after 30 months of ERT. The treatment was well tolerated without any adverse events.
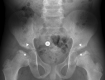
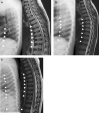
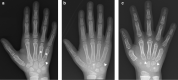
Conclusions: This case series highlights the importance of early recognition of the clinical and imaging findings that are initially subtle in MPS IVA. Early treatment with ERT is necessary to slow irreversible disease progression and improve patient outcomes. The oldest sibling experienced improvements in mobility despite severe symptoms resulting from a late diagnosis. When evaluating patients with skeletal anomalies, imaging multiple body regions is recommended. When findings such as anterior beaking of vertebrae or bilateral femoral head dysplasia are present, MPS IVA should be included in the differential diagnosis. Newborn screening must be considered for early detection, accurate diagnosis, and initiation of treatment to reduce morbidity.
Keywords: Anterior beaking; Diagnosis; Enzyme replacement therapy; Mucopolysaccharidosis IVA; Platyspondyly; Treatment.
Conflict of interest statement
Can Ficicioglu, MD, PhD, has served as an advisor or consultant for: Genzyme, Horizon, Orphan Tech., Recordati, Shire, and Sobi. He received grants for clinical research from: Genzyme, Orphan Tec, RegenxBio, Takeda, and Vtesse. Dena R. Matalon, MD, none. Nicole Luongo, PA-C, none. Caitlin Menello, CGC,, none. Tracy Kornafel,, none. Andrew J. Degnan, MD, none.
Figures



Similar articles
-
Enzyme replacement therapy attenuates disease progression in two Japanese siblings with mucopolysaccharidosis type VI.Mol Genet Metab. 2011 Dec;104(4):597-602. doi: 10.1016/j.ymgme.2011.08.029. Epub 2011 Aug 28. Mol Genet Metab. 2011. PMID: 21930407
-
Elosulfase alfa.Drugs Today (Barc). 2014 Jul;50(7):475-83. doi: 10.1358/dot.2014.50.7.2177904. Drugs Today (Barc). 2014. PMID: 25101330 Review.
-
Elosulfase Alfa: a review of its use in patients with mucopolysaccharidosis type IVA (Morquio A syndrome).BioDrugs. 2014 Oct;28(5):465-75. doi: 10.1007/s40259-014-0108-z. BioDrugs. 2014. PMID: 25200032 Review.
-
Clinical characteristics and effects of enzyme replacement therapy with elosulfase alfa in Korean patients with mucopolysaccharidosis type IVA.Mol Genet Metab Rep. 2022 Apr 15;31:100869. doi: 10.1016/j.ymgmr.2022.100869. eCollection 2022 Jun. Mol Genet Metab Rep. 2022. PMID: 35782601 Free PMC article.
-
Impact of enzyme replacement therapy and hematopoietic stem cell transplantation in patients with Morquio A syndrome.Drug Des Devel Ther. 2015 Apr 1;9:1937-53. doi: 10.2147/DDDT.S68562. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25897204 Free PMC article. Review.
Cited by
-
Mucopolysaccharidosis IVA: Current Disease Models and Drawbacks.Int J Mol Sci. 2023 Nov 9;24(22):16148. doi: 10.3390/ijms242216148. Int J Mol Sci. 2023. PMID: 38003337 Free PMC article. Review.
-
Molecular basis of mucopolysaccharidosis IVA (Morquio A syndrome): A review and classification of GALNS gene variants and reporting of 68 novel variants.Hum Mutat. 2021 Nov;42(11):1384-1398. doi: 10.1002/humu.24270. Epub 2021 Aug 23. Hum Mutat. 2021. PMID: 34387910 Free PMC article. Review.
-
Real-world treatment with elosulfase alfa in patients with MPS IVA is associated with improved endurance over time.Genet Med Open. 2025 Mar 29;3:103428. doi: 10.1016/j.gimo.2025.103428. eCollection 2025. Genet Med Open. 2025. PMID: 40677308 Free PMC article.
-
A systematic literature review of the impact and measurement of mobility impairment in rare bone diseases.Ther Adv Musculoskelet Dis. 2025 Aug 21;17:1759720X251369963. doi: 10.1177/1759720X251369963. eCollection 2025. Ther Adv Musculoskelet Dis. 2025. PMID: 40862204 Free PMC article. Review.
-
Delayed diagnosis of mild mucopolysaccharidosis type IVA.BMC Med Genomics. 2024 Jun 3;17(1):151. doi: 10.1186/s12920-024-01910-x. BMC Med Genomics. 2024. PMID: 38831290 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

