Analysis of long-term oncological results of clinical versus pathological responses after neoadjuvant treatment in locally advanced rectal cancer
- PMID: 33256819
- PMCID: PMC7706260
- DOI: 10.1186/s12957-020-02094-1
Analysis of long-term oncological results of clinical versus pathological responses after neoadjuvant treatment in locally advanced rectal cancer
Abstract
Background: Nonoperative management after neoadjuvant treatment in low rectal cancer enables organ preservation and avoids surgical morbidity. Our aim is to compare oncological outcomes in patients with clinical complete response in watch and wait strategy with those who received neoadjuvant therapy followed by surgery with a pathological complete response.
Methods: Patients with non-metastatic rectal cancer after neoadjuvant treatment with clinical complete response in watch and wait approach (group 1, n = 26) and complete pathological responders (ypT0N0) after chemoradiotherapy and surgery (group 2, n = 22), between January 2011 and October 2018, were included retrospectively, and all of them evaluated and followed in a multidisciplinary team. A comparative analysis of local and distant recurrence rates and disease-free and overall survival between both groups was carried out. Statistical analysis was performed using log-rank test, Cox proportional hazards regression model, and Kaplan-Meier curves.
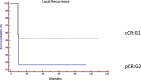
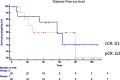
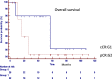
Results: No differences were found between patient's demographic characteristics in both groups. Group 1: distance from the anal verge mean 5 cm (r = 1-12), 10 (38%) stage III, and 7 (27%) circumferential resection margin involved. The median follow-up of 47 months (r = 6, a 108). Group 2: distance from the anal verge mean 7 cm (r = 2-12), 16 (72%) stage III, and 13 (59%) circumferential resection margin involved. The median follow-up 49.5 months (r = 3, a 112). Local recurrence: 2 patients in group 1 (8.3%) and 1 in group 2 (4.8%) (p = 0.6235). Distant recurrence: 1 patient in group 1 (3.8%) and 3 in group 2 (19.2%) (p = 0.2237). Disease-free survival: 87.9% in group 1, 80% in group 2 (p = 0.7546). Overall survival: 86% in group 1 and 85% in group 2 (p = 0.5367).
Conclusion: Oncological results in operated patients with pathological complete response were similar to those in patients under a watch and wait strategy mediating a systematic and personalized evaluation. Surgery can safely be deferred in clinical complete responders.
Keywords: Clinical complete response; Neoadjuvant treatment; Pathological complete response; Rectal cancer; Watch and wait.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, et al. Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total. BMC Cancer. 2015;15:1–13. doi: 10.1186/s12885-015-1632-z. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

