Mesenchymal stem cells and acellular products attenuate murine induced colitis
- PMID: 33256827
- PMCID: PMC7706051
- DOI: 10.1186/s13287-020-02025-7
Mesenchymal stem cells and acellular products attenuate murine induced colitis
Abstract
Background: Mesenchymal stem cells (MSCs) are a well-established immunomodulatory agent which can also promote tissue repair and regeneration. Recent studies have demonstrated MSCs as a novel therapeutic for inflammatory bowel disease (IBD), a chronic idiopathic inflammatory disorder of the gastrointestinal tract. However, the precise role of MSCs in regulating immune responses is controversial, and its significance in the pathogenesis remains IBD undefined. In addition, MSCs' acellular product, extracellular vesicles (EVs), may also play an important role in the armamentarium of therapeutics, but how EVs compare to MSCs remains unknown due to the lack of side-by-side comparative investigation. We herein compared MSCs and MSC-derived EVs for the treatment of IBD using a DSS-induced colitis model.
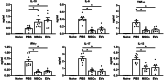
Methods: A DSS-induced colitis model was used. At day 4, mice received adipose-derived MSCs, MSC-derived EVs, or placebo. Weight loss, stool consistency, and hematochezia was charted. At day 8, murine colons were harvested, histologic analysis performed, and serum/tissue cytokine analysis conducted.
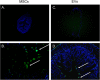
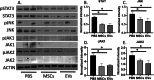
Results: MSCs and EVs demonstrated equivalent immunosuppressive function in DSS-treated mice through decreased colonic lymphocyte infiltration and attenuated disease severity after both MSC and EV treatment. Furthermore, both MSCs and EVs have an equivalent ability to inhibit inflammation in the DSS colitis model by inhibiting JAK, JNK 1/2, and STAT3 signaling.
Conclusions: These results suggest that (i) both MSCs and EVs are effective therapeutic candidates for a DSS-induced mouse colitis model, (ii) MSCs and EVs have similar immunosuppressive and anti-inflammatory functions, and (iii) EVs may present a novel future therapeutic for the treatment of IBD.
Keywords: Extracellular vesicles; Inflammatory bowel disease; Mesenchymal stem cells; Murine colitis model; Therapy.
Conflict of interest statement
Amy Lightner declares the consult of Takeda. Other authors declare that there is no conflict of interest regarding the publication of this article.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

