Comparative effectiveness of direct admission and admission through emergency departments for children: a randomized stepped wedge study protocol
- PMID: 33256850
- PMCID: PMC7706271
- DOI: 10.1186/s13063-020-04889-9
Comparative effectiveness of direct admission and admission through emergency departments for children: a randomized stepped wedge study protocol
Abstract
Background: Approximately 2 million children are hospitalized each year in the United States, with more than three-quarters of non-elective hospitalizations admitted through emergency departments (EDs). Direct admission, defined as admission to hospital without first receiving care in the hospital's ED, may offer benefits for patients and healthcare systems in quality, timeliness, and experience of care. While ED utilization patterns are well studied, there is a paucity of research comparing the effectiveness of direct and ED admissions. The overall aim of this project is to compare the effectiveness of a standardized direct admission approach to admission beginning in the ED for hospitalized children.
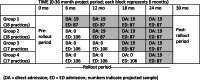
Methods/design: We will conduct a stepped wedge cluster randomized controlled trial at 3 structurally and geographically diverse hospitals. A total of 70 primary and urgent care practice sites in the hospitals' catchment areas will be randomized to a time point when they will begin participation in the multi-stakeholder informed direct admission program. This crossover will be unidirectional and occur at 4 time points, 6 months apart, over a 24-month implementation period. Our primary outcome will be the timeliness of clinical care provision. Secondary outcomes include (i) parent-reported experience of care, (ii) unanticipated transfer to the intensive care unit within 6 h of hospital admission, and (iii) rapid response calls within 6 h of hospital admission. We anticipate that 190 children and adolescents will be directly admitted, with 1506 admitted through EDs. Analyses will compare the effectiveness of direct admission to admission through the ED and will evaluate the causal effect of implementing a direct admission program using linear regression with random effects for referring practice clusters and time period fixed effects. We will further examine the heterogeneity of treatment effects based on hypotheses specified a priori. In addition, we will conduct a mixed-methods process evaluation to assess reach, effectiveness, adoption, implementation, and maintenance of our direct admission intervention.
Discussion: Our study represents the first randomized controlled trial to compare the effectiveness of direct admission to admission through the ED for pediatric patients. Our scientific approach, pairing a stepped wedge design with a multi-level assessment of barriers to and facilitators of implementation, will generate valuable data about how positive findings can be reproduced across other healthcare systems.
Trial registration: ClinicalTrials.gov NCT04192799 . Registered on December 10, 2019).
Keywords: Cluster randomized controlled trial; Direct admission; Implementation; Multi-stakeholder teams; Pediatric hospital medicine; Pediatric hospitalizations.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Assessing effectiveness and implementation of a perioperative enhanced recovery protocol for children undergoing surgery: study protocol for a prospective, stepped-wedge, cluster, randomized, controlled clinical trial.Trials. 2020 Nov 16;21(1):926. doi: 10.1186/s13063-020-04851-9. Trials. 2020. PMID: 33198767 Free PMC article.
-
Protocol for a multi-centered, stepped wedge, cluster randomized controlled trial of the de-adoption of oral chlorhexidine prophylaxis and implementation of an oral care bundle for mechanically ventilated critically ill patients: the CHORAL study.Trials. 2019 Oct 24;20(1):603. doi: 10.1186/s13063-019-3673-0. Trials. 2019. PMID: 31651364 Free PMC article.
-
Effectiveness of Direct Admission Compared to Admission Through the Emergency Department: A Stepped-Wedge Cluster-Randomized Trial.Pediatrics. 2024 Oct 1;154(4):e2024065776. doi: 10.1542/peds.2024-065776. Pediatrics. 2024. PMID: 39301600 Clinical Trial.
-
Knowledge to action: Rationale and design of the Patient-Centered Care Transitions in Heart Failure (PACT-HF) stepped wedge cluster randomized trial.Am Heart J. 2018 May;199:75-82. doi: 10.1016/j.ahj.2017.12.013. Epub 2017 Dec 27. Am Heart J. 2018. PMID: 29754670 Review.
-
Does an Advanced Electronic Tracker Help Families Manage Children's Asthma Symptoms Better Than a Standard Electronic Tracker? [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Oct. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Oct. PMID: 38683906 Free Books & Documents. Review.
References
-
- Lassman D, Hartman M, Washington B, Andrews K, Catlin A. US health spending trends by age and gender: selected years 2002–10. Health Aff (Millwood). 2014;33(5):815–22. 10.1377/hlthaff.2013.1224. - PubMed
-
- Moore B, Levit K, Elixhauser A. Costs for Hospital Stays in the United States, 2012: Statistical Brief #181. 2014 Oct. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville: Agency for Healthcare Research and Quality (US); 2006. Available from: https://www.ncbi.nlm.nih.gov/books/NBK259217/. - PubMed
-
- Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus Policy Statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976. doi: 10.1007/s11606-009-0969-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

