Immunosuppression in chronic autoimmune neurological disorders during the COVID-19 pandemic
- PMID: 33256952
- PMCID: PMC7837234
- DOI: 10.1016/j.jns.2020.117230
Immunosuppression in chronic autoimmune neurological disorders during the COVID-19 pandemic
Abstract
Objective: To study the risk of acquiring Corona Virus Disease 2019 (COVID-19) and its outcomes in patients on immunosuppressive therapy (IST) for chronic autoimmune neuromuscular disorders (aNMD) and multiple sclerosis (MS).
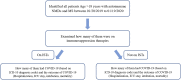
Methods: We used TriNetX, a global health collaborative clinical research platform collecting real-time electronic medical records data, which has one of the largest known global COVID-19 database. We included patients with chronic autoimmune neuromuscular disorders (aNMD) [myasthenia gravis (MG), inflammatory myositis, and chronic inflammatory neuropathies (CIN)] and MS, based on the International Classification of Disease-10 (ICD-10) coding for one year before January 20th, 2020. We examined the use of IST, rate of COVID- 19, hospitalization, intubation, and mortality among the patients with aNMD and MS.
Results: A total of 33,451 patients with aNMD and 42,899 patients with MS were included. Among them, 111 (0.33%) patients with aNMD and 115 patients (0.27%) with MS had COVID-19. About one third of them required hospitalization. IST did not appear to have a significant impact on overall infection risk in either group; however, risk of hospitalization for immunosuppressed patients with aNMD was higher (Odds ratio 2.86, p-value 0.011).
Conclusions: IST use does not appear to make patients with aNMD and MS more vulnerable to COVID-19. IST may be continued during the pandemic, as previously suggested by expert opinion guidelines. However, it is important to consider individualizing immunotherapy regimens in some cases. Additional physician reported registry-based data is needed to further confirm these findings.
Keywords: Autoimmune disease; COVID-19; Immunosuppressive therapies; Multiple sclerosis; Neurological disorders; Neuromuscular disorders.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Risk and course of COVID-19 in immunosuppressed patients with myasthenia gravis.J Neurol. 2023 Jan;270(1):1-12. doi: 10.1007/s00415-022-11389-0. Epub 2022 Sep 27. J Neurol. 2023. PMID: 36166068 Free PMC article.
-
Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis.Ann Neurol. 2021 Apr;89(4):780-789. doi: 10.1002/ana.26028. Epub 2021 Feb 9. Ann Neurol. 2021. PMID: 33480077 Free PMC article.
-
Associations of Disease-Modifying Therapies With COVID-19 Severity in Multiple Sclerosis.Neurology. 2021 Nov 9;97(19):e1870-e1885. doi: 10.1212/WNL.0000000000012753. Epub 2021 Oct 5. Neurology. 2021. PMID: 34610987 Free PMC article.
-
Impact of disease-modifying drugs on the severity of COVID-19 infection in multiple sclerosis patients.J Med Virol. 2021 Mar;93(3):1314-1319. doi: 10.1002/jmv.26593. Epub 2020 Oct 30. J Med Virol. 2021. PMID: 33044760 Free PMC article. Review.
-
Neuromuscular diseases associated with chronic hepatitis C virus infection.J Clin Neuromuscul Dis. 2011 Sep;13(1):14-25. doi: 10.1097/CND.0b013e3181d4a527. J Clin Neuromuscul Dis. 2011. PMID: 22361622 Review.
Cited by
-
To Be or Not To Be Vaccinated: That Is a Question in Myasthenia Gravis.Front Immunol. 2021 Sep 17;12:733418. doi: 10.3389/fimmu.2021.733418. eCollection 2021. Front Immunol. 2021. PMID: 34603311 Free PMC article. Review.
-
Systematic review of risk of SARS-CoV-2 infection and severity of COVID-19 with therapies approved to treat multiple sclerosis.Neurol Sci. 2022 Mar;43(3):1557-1567. doi: 10.1007/s10072-021-05846-3. Epub 2022 Jan 10. Neurol Sci. 2022. PMID: 35006442 Free PMC article.
-
Risk factors of severe COVID-19 in people with multiple sclerosis : A systematic review and meta-analysis.Rev Neurol (Paris). 2022 Jan-Feb;178(1-2):121-128. doi: 10.1016/j.neurol.2021.10.003. Epub 2021 Nov 4. Rev Neurol (Paris). 2022. PMID: 34836608 Free PMC article.
-
Neuromuscular complications of coronavirus disease-19.Curr Opin Neurol. 2021 Oct 1;34(5):669-674. doi: 10.1097/WCO.0000000000000970. Curr Opin Neurol. 2021. PMID: 34155186 Free PMC article. Review.
-
Knowledge, attitudes, and impact of COVID-19 pandemic among neurology patients in Jordan: a cross-sectional study.Egypt J Neurol Psychiatr Neurosurg. 2021;57(1):104. doi: 10.1186/s41983-021-00354-9. Epub 2021 Jul 29. Egypt J Neurol Psychiatr Neurosurg. 2021. PMID: 34341652 Free PMC article.
References
-
- COVID-19. World Health Organization; 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical