Heterogeneity of Age-Related Neural Hyperactivity along the CA3 Transverse Axis
- PMID: 33257325
- PMCID: PMC7842755
- DOI: 10.1523/JNEUROSCI.2405-20.2020
Heterogeneity of Age-Related Neural Hyperactivity along the CA3 Transverse Axis
Abstract
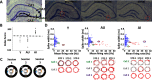
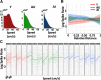
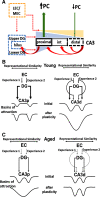
Age-related memory deficits are correlated with neural hyperactivity in the CA3 region of the hippocampus. Abnormal CA3 hyperactivity in aged rats has been proposed to contribute to an imbalance between pattern separation and pattern completion, resulting in overly rigid representations. Recent evidence of functional heterogeneity along the CA3 transverse axis suggests that proximal CA3 supports pattern separation while distal CA3 supports pattern completion. It is not known whether age-related CA3 hyperactivity is uniformly represented along the CA3 transverse axis. We examined the firing rates of CA3 neurons from young and aged, male, Long-Evans rats along the CA3 transverse axis. Consistent with prior studies, young CA3 cells showed an increasing gradient in mean firing rate from proximal to distal CA3. However, aged CA3 cells showed an opposite, decreasing trend, in that CA3 cells in aged rats were hyperactive in proximal CA3, but possibly hypoactive in distal CA3, compared with young (Y) rats. We suggest that, in combination with altered inputs from the entorhinal cortex and dentate gyrus (DG), the proximal CA3 region of aged rats may switch from its normal function that reflects the pattern separation output of the DG and instead performs a computation that reflects an abnormal bias toward pattern completion. In parallel, distal CA3 of aged rats may create weaker attractor basins that promote abnormal, bistable representations under certain conditions.SIGNIFICANCE STATEMENT Prior work suggested that age-related CA3 hyperactivity enhances pattern completion, resulting in rigid representations. Implicit in prior studies is the notion that hyperactivity is present throughout a functionally homogeneous CA3 network. However, more recent work has demonstrated functional heterogeneity along the CA3 transverse axis, in that proximal CA3 is involved in pattern separation and distal CA3 is involved in pattern completion. Here, we show that age-related hyperactivity is present only in proximal CA3, with potential hypoactivity in distal CA3. This result provides new insight in the role of CA3 in age-related memory impairments, suggesting that the rigid representations in aging result primarily from dysfunction of computational circuits involving the dentate gyrus (DG) and proximal CA3.
Keywords: CA3; aging; hippocampus; hyperactivity; pattern completion; pattern separation.
Copyright © 2021 the authors.
Figures



References
-
- Aitchison J, Silvey SD (1960) Maximum-likelihood estimation procedures and associated tests of significance. J R Stat Soc Series B Stat Methodol 22:154–171. 10.1111/j.2517-6161.1960.tb00362.x - DOI
-
- Amaral D, Lavenex P (2007) Hippocampal neuroanatomy. In: The hippocampus book. Oxford: Oxford University Press.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
