Beneficial and harmful effects of sacubitril/valsartan in patients with heart failure: a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis
- PMID: 33257469
- PMCID: PMC7705560
- DOI: 10.1136/openhrt-2020-001294
Beneficial and harmful effects of sacubitril/valsartan in patients with heart failure: a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis
Abstract
Current guidelines recommend angiotensin receptor blocker neprilysin inhibitors (ARNI) (sacubitril/valsartan) as a replacement for angiotensin-converting-enzymeinhibitor (ACE-I) in heart failure with reduced ejection fraction (HFrEF) who remain symptomatic despite optimal medical therapy. The effects of ARNIs have not previously been assessed in a systematic review. We searched for relevant trials until October 2019 in CENTRAL, MEDLINE, Embase, LILACS, BIOSIS, CNKI, VIP, WanFang and CBM. Our primary outcomes were all-cause mortality and serious adverse events. We systematically assessed the risks of random errors and systematic errors. PROSPERO registration: CRD42019129336. 48 trials randomising 19 086 participants were included. The ARNI assessed in all trials was sacubitril/valsartan. ACE-I or ARB were used as control interventions. Trials randomising HFrEF participants (27 trials) and heart failure with preserved ejection fraction (HFpEF) participants (four trials) were analysed separately. In HFrEF participants, meta-analyses and Trial Sequential Analyses showed evidence of a beneficial effect of sacubitril/valsartan when assessing all-cause mortality (risk ratio (RR), 0.86; 95% CI, 0.79 to 0.94) and serious adverse events (RR, 0.89; 95% CI, 0.86 to 0.93); and the results did not differ between the guideline recommended target population and HFrEF participants in general. We found no evidence of an effect of sacubitril/valsartan in HFpEF participants. Sacubitril/valsartan compared with either ACE-I or ARB seems to have a beneficial effect in patients with HFrEF. Our results indicate that sacubitril/valsartan might be beneficial in a wider population of patients with heart failure than the guideline recommended target population. Sacubitril/valsartan does not seem to show evidence of a difference compared with valsartan in patients with HFpEF.
Keywords: heart failure; heart failure treatment; renin-angiotensin system.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




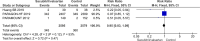

Similar articles
-
Effect of sacubitril/valsartan and ACEI/ARB on glycaemia and the development of diabetes: a systematic review and meta-analysis of randomised controlled trials.BMC Med. 2022 Dec 17;20(1):487. doi: 10.1186/s12916-022-02682-w. BMC Med. 2022. PMID: 36527023 Free PMC article.
-
Progress and prospects of Sacubitril/Valsartan: Based on heart failure with preserved ejection fraction.Biomed Pharmacother. 2022 Nov;155:113701. doi: 10.1016/j.biopha.2022.113701. Epub 2022 Sep 15. Biomed Pharmacother. 2022. PMID: 36116249 Review.
-
Comparative effects of sacubitril/valsartan and ACEI/ARB on endothelial function and arterial stiffness in patients with heart failure: a protocol for systematic review and meta-analysis.BMJ Open. 2024 Sep 10;14(9):e088744. doi: 10.1136/bmjopen-2024-088744. BMJ Open. 2024. PMID: 39260836 Free PMC article.
-
The Efficacy and Safety of Sacubitril/Valsartan in Heart Failure Patients: A Review.J Cardiovasc Pharmacol Ther. 2022 Jan-Dec;27:10742484211058681. doi: 10.1177/10742484211058681. J Cardiovasc Pharmacol Ther. 2022. PMID: 34994233 Review.
-
Angiotensin Receptor Neprilysin Inhibition in Heart Failure With Preserved Ejection Fraction: Rationale and Design of the PARAGON-HF Trial.JACC Heart Fail. 2017 Jul;5(7):471-482. doi: 10.1016/j.jchf.2017.04.013. Epub 2017 Jun 26. JACC Heart Fail. 2017. PMID: 28662936 Review.
Cited by
-
Network Meta-Analysis Comparing Angiotensin Receptor-Neprilysin Inhibitors, Angiotensin Receptor Blockers, and Angiotensin-Converting Enzyme Inhibitors in Heart Failure With Reduced Ejection Fraction.Am J Cardiol. 2023 Jan 15;187:84-92. doi: 10.1016/j.amjcard.2022.10.026. Epub 2022 Nov 29. Am J Cardiol. 2023. PMID: 36459752 Free PMC article.
-
Sacubitril/valsartan in a wide spectrum of heart failure patients (from mechanisms of action to outcomes in specific populations).Heart Fail Rev. 2025 Mar;30(2):387-405. doi: 10.1007/s10741-024-10471-1. Epub 2025 Jan 7. Heart Fail Rev. 2025. PMID: 39776087 Free PMC article. Review.
-
The Efficacy and Safety of Sacubitril/Valsartan Compared to Valsartan in Patients with Heart Failure and Mildly Reduced and Preserved Ejection Fractions: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.J Clin Med. 2024 Mar 9;13(6):1572. doi: 10.3390/jcm13061572. J Clin Med. 2024. PMID: 38541798 Free PMC article. Review.
-
Beneficial and harmful effects of tricyclic antidepressants for adults with major depressive disorder: a systematic review with meta-analysis and trial sequential analysis.BMJ Ment Health. 2024 Jan 22;27(1):e300730. doi: 10.1136/bmjment-2023-300730. BMJ Ment Health. 2024. PMID: 39093721 Free PMC article.
-
Benefits and adverse effects of sacubitril/valsartan in patients with chronic heart failure: A systematic review and meta-analysis.Pharmacol Res Perspect. 2021 Oct;9(5):e00844. doi: 10.1002/prp2.844. Pharmacol Res Perspect. 2021. PMID: 34617669 Free PMC article.
References
-
- WRITING COMMITTEE MEMBERS, Yancy CW, Jessup M, et al. . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines. Circulation 2013;128:e240–327. 10.1161/CIR.0b013e31829e8776 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
