Innate IFN-γ Is Essential for Systemic Chlamydia muridarum Control in Mice, While CD4 T Cell-Dependent IFN-γ Production Is Highly Redundant in the Female Reproductive Tract
- PMID: 33257535
- PMCID: PMC8097277
- DOI: 10.1128/IAI.00541-20
Innate IFN-γ Is Essential for Systemic Chlamydia muridarum Control in Mice, While CD4 T Cell-Dependent IFN-γ Production Is Highly Redundant in the Female Reproductive Tract
Abstract
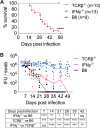
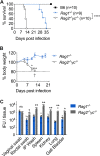
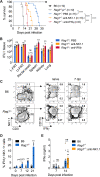
Protective immunity against the obligate intracellular bacterium Chlamydia has long been thought to rely on CD4 T cell-dependent gamma interferon (IFN-γ) production. Nevertheless, whether IFN-γ is produced by other cellular sources during Chlamydia infection and how CD4 T cell-dependent and -independent IFN-γ contribute differently to host resistance have not been carefully evaluated. In this study, we dissected the requirements of IFN-γ produced by innate immune cells and CD4 T cells for resolution of Chlamydia muridarum female reproductive tract (FRT) infection. After C. muridarum intravaginal infection, IFN-γ-deficient and T cell-deficient mice exhibited opposite phenotypes for survival and bacterial shedding at the FRT mucosa, demonstrating the distinct requirements for IFN-γ and CD4 T cells in host defense against Chlamydia In Rag1-deficient mice, IFN-γ produced by innate lymphocytes (ILCs) accounted for early bacterial control and prolonged survival in the absence of adaptive immunity. Although type I ILCs are potent IFN-γ producers, we found that mature NK cells and ILC1s were not the sole sources of innate IFN-γ in response to Chlamydia By conducting T cell adoptive transfer, we showed definitively that IFN-γ-deficient CD4 T cells were sufficient for effective bacterial killing in the FRT during the first 21 days of infection and reduced bacterial burden more than 1,000-fold, although mice receiving IFN-γ-deficient CD4 T cells failed to completely eradicate the bacteria from the FRT like their counterparts receiving wild-type (WT) CD4 T cells. Together, our results revealed that innate IFN-γ is essential for preventing systemic Chlamydia dissemination, whereas IFN-γ produced by CD4 T cells is largely redundant at the FRT mucosa.
Keywords: CD4 T cells; Chlamydia; IFN-γ; infection; innate.
Copyright © 2021 American Society for Microbiology.
Figures

Similar articles
-
Immunopathogenesis of genital Chlamydia infection: insights from mouse models.Pathog Dis. 2021 Mar 31;79(4):ftab012. doi: 10.1093/femspd/ftab012. Pathog Dis. 2021. PMID: 33538819 Free PMC article. Review.
-
T Cell-Independent Gamma Interferon and B Cells Cooperate To Prevent Mortality Associated with Disseminated Chlamydia muridarum Genital Tract Infection.Infect Immun. 2018 Jun 21;86(7):e00143-18. doi: 10.1128/IAI.00143-18. Print 2018 Jul. Infect Immun. 2018. PMID: 29661927 Free PMC article.
-
Antigen-specific CD4+ T cells produce sufficient IFN-gamma to mediate robust protective immunity against genital Chlamydia muridarum infection.J Immunol. 2008 Mar 1;180(5):3375-82. doi: 10.4049/jimmunol.180.5.3375. J Immunol. 2008. PMID: 18292563
-
Adoptive Transfer of Group 3-Like Innate Lymphoid Cells Restores Mouse Colon Resistance to Colonization of a Gamma Interferon-Susceptible Chlamydia muridarum Mutant.Infect Immun. 2021 Jan 19;89(2):e00533-20. doi: 10.1128/IAI.00533-20. Print 2021 Jan 19. Infect Immun. 2021. PMID: 33139384 Free PMC article.
-
Diversity in the T cell response to Chlamydia-sum are better than one.Immunol Lett. 2018 Oct;202:59-64. doi: 10.1016/j.imlet.2018.08.002. Epub 2018 Sep 1. Immunol Lett. 2018. PMID: 30179654 Free PMC article. Review.
Cited by
-
Microbiota metabolites in the female reproductive system: Focused on the short-chain fatty acids.Heliyon. 2023 Mar 14;9(3):e14562. doi: 10.1016/j.heliyon.2023.e14562. eCollection 2023 Mar. Heliyon. 2023. PMID: 36967966 Free PMC article. Review.
-
Murine modeling of menstruation identifies immune correlates of protection during Chlamydia muridarum challenge.PLoS Pathog. 2025 Jun 6;21(6):e1012276. doi: 10.1371/journal.ppat.1012276. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40478914 Free PMC article.
-
Innate Lymphoid Cells in Response to Intracellular Pathogens: Protection Versus Immunopathology.Front Cell Infect Microbiol. 2021 Dec 6;11:775554. doi: 10.3389/fcimb.2021.775554. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34938670 Free PMC article. Review.
-
Immunopathogenesis of genital Chlamydia infection: insights from mouse models.Pathog Dis. 2021 Mar 31;79(4):ftab012. doi: 10.1093/femspd/ftab012. Pathog Dis. 2021. PMID: 33538819 Free PMC article. Review.
-
Elimination of Chlamydia muridarum from the female reproductive tract is IL-12p40 dependent, but independent of Th1 and Th2 cells.PLoS Pathog. 2024 Jan 2;20(1):e1011914. doi: 10.1371/journal.ppat.1011914. eCollection 2024 Jan. PLoS Pathog. 2024. PMID: 38166152 Free PMC article.
References
-
- Morré SA, Brule A, Rozendaal L, Boeke AJP, Voorhorst FJ, Blok SD, Meijer CJLM. 2002. The natural course of asymptomatic Chlamydia trachomatis infections: 45% clearance and no development of clinical PID after one-year follow-up. Int J STD AIDS 13:12–18. doi:10.1258/095646202762226092. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

