STAT3 Promotes Schistosome-Induced Liver Injury by Inflammation, Oxidative Stress, Proliferation, and Apoptosis Signal Pathway
- PMID: 33257536
- PMCID: PMC8097265
- DOI: 10.1128/IAI.00309-20
STAT3 Promotes Schistosome-Induced Liver Injury by Inflammation, Oxidative Stress, Proliferation, and Apoptosis Signal Pathway
Abstract
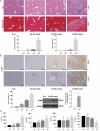
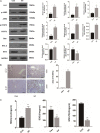
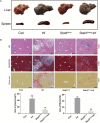
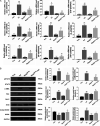
Schistosomiasis is a parasitic helminth disease that can cause organ lesions leading to health damage. During a schistosome infection, schistosome eggs can flow into the liver along the portal vein. Numerous inflammatory cells gather around the eggs, causing granulomas and fibrosis in the liver. In this process, many molecules are involved in the initiation and regulation of the fibrous scar formation. However, the precise molecular mechanisms responsible for the progression of granuloma formation and fibrosis initiation caused by schistosome infection have not been extensively studied. In this study, C57BL/6 wild-type mice and Stat3flox/flox Alb-Cre mice were infected with cercariae of Schistosoma japonicum Liver injury, effector molecule levels, and RNA transcriptome resequencing of liver tissue were detected at 4, 5, and 6 weeks postinfection. We investigated the role of STAT3 (signal transducer and activator of transcription 3) in Schistosoma-induced liver injury in mice. After 6 weeks postinfection, there was obvious liver fibrosis. A sustained pathological process (inflammation, oxidative stress, proliferation, and apoptosis) occurred in S. japonicum-induced liver fibrosis initiation. Meanwhile, we observed activation of the STAT3 pathway in hepatic injury during S. japonicum infection by RNA transcriptome resequencing. Liver deficiency of phospho-STAT3 alleviated infection-induced liver dysfunction, hepatic granuloma formation, and fibrosis initiation. It also promoted STAT3-dependent apoptosis and reduced liver inflammation, oxidative stress, and proliferation. Our results suggest that STAT3 signal pathway and its mediating inflammation, oxidative stress, proliferation, and apoptosis are involved in S. japonicum-induced liver injury and may be a new potential guideline for the treatment of schistosomiasis.
Keywords: S. japonicum; STAT3; apoptosis; inflammation; liver injury; oxidative stress; proliferation.
Copyright © 2021 American Society for Microbiology.
Figures

Similar articles
-
Pathology and molecular mechanisms of Schistosoma japonicum-associated liver fibrosis.Front Cell Infect Microbiol. 2022 Oct 28;12:1035765. doi: 10.3389/fcimb.2022.1035765. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36389166 Free PMC article. Review.
-
Taurine Alleviates Schistosoma-Induced Liver Injury by Inhibiting the TXNIP/NLRP3 Inflammasome Signal Pathway and Pyroptosis.Infect Immun. 2019 Nov 18;87(12):e00732-19. doi: 10.1128/IAI.00732-19. Print 2019 Dec. Infect Immun. 2019. PMID: 31570558 Free PMC article.
-
A boswellic acid-containing extract ameliorates schistosomiasis liver granuloma and fibrosis through regulating NF-κB signaling in mice.PLoS One. 2014 Jun 18;9(6):e100129. doi: 10.1371/journal.pone.0100129. eCollection 2014. PLoS One. 2014. PMID: 24941000 Free PMC article.
-
MiR-383-5p promotes schistosomiasis-induced liver fibrosis by targeting peroxiredoxin-3.Parasit Vectors. 2025 Jun 3;18(1):205. doi: 10.1186/s13071-025-06824-w. Parasit Vectors. 2025. PMID: 40462224 Free PMC article.
-
Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology.Trends Parasitol. 2014 Mar;30(3):141-50. doi: 10.1016/j.pt.2013.12.009. Epub 2014 Jan 13. Trends Parasitol. 2014. PMID: 24433721 Review.
Cited by
-
Aqueous extract of Amydrium sinense (Engl.) H. Li alleviates hepatic fibrosis by suppressing hepatic stellate cell activation through inhibiting Stat3 signaling.Front Pharmacol. 2023 Jun 13;14:1101703. doi: 10.3389/fphar.2023.1101703. eCollection 2023. Front Pharmacol. 2023. PMID: 37383718 Free PMC article.
-
Differential Analysis of Key Proteins Related to Fibrosis and Inflammation in Soluble Egg Antigen of Schistosoma mansoni at Different Infection Times.Pathogens. 2023 Mar 11;12(3):441. doi: 10.3390/pathogens12030441. Pathogens. 2023. PMID: 36986363 Free PMC article.
-
Dysregulated Glucuronidation of Bilirubin Exacerbates Liver Inflammation and Fibrosis in Schistosomiasis Japonica through the NF-κB Signaling Pathway.Pathogens. 2024 Mar 28;13(4):287. doi: 10.3390/pathogens13040287. Pathogens. 2024. PMID: 38668242 Free PMC article.
-
Aerobic Exercise Ameliorates Liver Injury in Db/Db Mice by Attenuating Oxidative Stress, Apoptosis and Inflammation Through the Nrf2 and JAK2/STAT3 Signalling Pathways.J Inflamm Res. 2023 Oct 24;16:4805-4819. doi: 10.2147/JIR.S426581. eCollection 2023. J Inflamm Res. 2023. PMID: 37901382 Free PMC article.
-
Pathology and molecular mechanisms of Schistosoma japonicum-associated liver fibrosis.Front Cell Infect Microbiol. 2022 Oct 28;12:1035765. doi: 10.3389/fcimb.2022.1035765. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36389166 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

