Functional characterization of 67 endocytic accessory proteins using multiparametric quantitative analysis of CCP dynamics
- PMID: 33257546
- PMCID: PMC7749282
- DOI: 10.1073/pnas.2020346117
Functional characterization of 67 endocytic accessory proteins using multiparametric quantitative analysis of CCP dynamics
Abstract
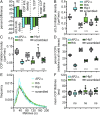
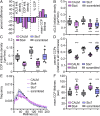
Clathrin-mediated endocytosis (CME) begins with the nucleation of clathrin assembly on the plasma membrane, followed by stabilization and growth/maturation of clathrin-coated pits (CCPs) that eventually pinch off and internalize as clathrin-coated vesicles. This highly regulated process involves a myriad of endocytic accessory proteins (EAPs), many of which are multidomain proteins that encode a wide range of biochemical activities. Although domain-specific activities of EAPs have been extensively studied, their precise stage-specific functions have been identified in only a few cases. Using single-guide RNA (sgRNA)/dCas9 and small interfering RNA (siRNA)-mediated protein knockdown, combined with an image-based analysis pipeline, we have determined the phenotypic signature of 67 EAPs throughout the maturation process of CCPs. Based on these data, we show that EAPs can be partitioned into phenotypic clusters, which differentially affect CCP maturation and dynamics. Importantly, these clusters do not correlate with functional modules based on biochemical activities. Furthermore, we discover a critical role for SNARE proteins and their adaptors during early stages of CCP nucleation and stabilization and highlight the importance of GAK throughout CCP maturation that is consistent with GAK's multifunctional domain architecture. Together, these findings provide systematic, mechanistic insights into the plasticity and robustness of CME.
Keywords: CRISPRi screen; GAK; SNAREs; clathrin-mediated endocytosis; total internal reflection fluorescence microscopy.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- McMahon H. T., Boucrot E., Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533 (2011). - PubMed
-
- Kaksonen M., Roux A., Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 19, 313–326 (2018). - PubMed
-
- Ehrlich M., et al. , Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591–605 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

