A bacterial cytolinker couples positioning of magnetic organelles to cell shape control
- PMID: 33257551
- PMCID: PMC7749328
- DOI: 10.1073/pnas.2014659117
A bacterial cytolinker couples positioning of magnetic organelles to cell shape control
Abstract
Magnetotactic bacteria maneuver within the geomagnetic field by means of intracellular magnetic organelles, magnetosomes, which are aligned into a chain and positioned at midcell by a dedicated magnetosome-specific cytoskeleton, the "magnetoskeleton." However, how magnetosome chain organization and resulting magnetotaxis is linked to cell shape has remained elusive. Here, we describe the cytoskeletal determinant CcfM (curvature-inducing coiled-coil filament interacting with the magnetoskeleton), which links the magnetoskeleton to cell morphology regulation in Magnetospirillum gryphiswaldense Membrane-anchored CcfM localizes in a filamentous pattern along regions of inner positive-cell curvature by its coiled-coil motifs, and independent of the magnetoskeleton. CcfM overexpression causes additional circumferential localization patterns, associated with a dramatic increase in cell curvature, and magnetosome chain mislocalization or complete chain disruption. In contrast, deletion of ccfM results in decreased cell curvature, impaired cell division, and predominant formation of shorter, doubled chains of magnetosomes. Pleiotropic effects of CcfM on magnetosome chain organization and cell morphology are supported by the finding that CcfM interacts with the magnetoskeleton-related MamY and the actin-like MamK via distinct motifs, and with the cell shape-related cytoskeleton via MreB. We further demonstrate that CcfM promotes motility and magnetic alignment in structured environments, and thus likely confers a selective advantage in natural habitats of magnetotactic bacteria, such as aquatic sediments. Overall, we unravel the function of a prokaryotic cytoskeletal constituent that is widespread in magnetic and nonmagnetic spirilla-shaped Alphaproteobacteria.
Keywords: MamK; MreB; cytoskeleton; magnetosome; magnetotaxis.
Conflict of interest statement
The authors declare no competing interest.
Figures
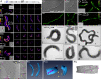
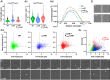
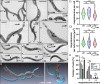
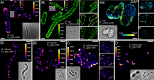



References
-
- Uebe R., Schüler D., Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 14, 621–637 (2016). - PubMed
-
- Komeili A., Li Z., Newman D. K., Jensen G. J., Magnetosomes are cell membrane invaginations organized by the actin-like protein MamK. Science 311, 242–245 (2006). - PubMed
-
- Katzmann E., Scheffel A., Gruska M., Plitzko J. M., Schüler D., Loss of the actin-like protein MamK has pleiotropic effects on magnetosome formation and chain assembly in Magnetospirillum gryphiswaldense. Mol. Microbiol. 77, 208–224 (2010). - PubMed
-
- Katzmann E., et al. , Magnetosome chains are recruited to cellular division sites and split by asymmetric septation. Mol. Microbiol. 82, 1316–1329 (2011). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

