A wireless, skin-interfaced biosensor for cerebral hemodynamic monitoring in pediatric care
- PMID: 33257558
- PMCID: PMC7749320
- DOI: 10.1073/pnas.2019786117
A wireless, skin-interfaced biosensor for cerebral hemodynamic monitoring in pediatric care
Abstract
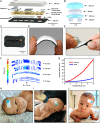
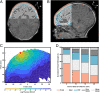
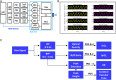
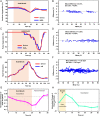
The standard of clinical care in many pediatric and neonatal neurocritical care units involves continuous monitoring of cerebral hemodynamics using hard-wired devices that physically adhere to the skin and connect to base stations that commonly mount on an adjacent wall or stand. Risks of iatrogenic skin injuries associated with adhesives that bond such systems to the skin and entanglements of the patients and/or the healthcare professionals with the wires can impede clinical procedures and natural movements that are critical to the care, development, and recovery of pediatric patients. This paper presents a wireless, miniaturized, and mechanically soft, flexible device that supports measurements quantitatively comparable to existing clinical standards. The system features a multiphotodiode array and pair of light-emitting diodes for simultaneous monitoring of systemic and cerebral hemodynamics, with ability to measure cerebral oxygenation, heart rate, peripheral oxygenation, and potentially cerebral pulse pressure and vascular tone, through the utilization of multiwavelength reflectance-mode photoplethysmography and functional near-infrared spectroscopy. Monte Carlo optical simulations define the tissue-probing depths for source-detector distances and operating wavelengths of these systems using magnetic resonance images of the head of a representative pediatric patient to define the relevant geometries. Clinical studies on pediatric subjects with and without congenital central hypoventilation syndrome validate the feasibility for using this system in operating hospitals and define its advantages relative to established technologies. This platform has the potential to substantially enhance the quality of pediatric care across a wide range of conditions and use scenarios, not only in advanced hospital settings but also in clinics of lower- and middle-income countries.
Keywords: bioelectronics; cerebral hemodynamics; near-infrared spectroscopy; wearable electronics.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Carroll M. S., et al. , Residual chemosensitivity to ventilatory challenges in genotyped congenital central hypoventilation syndrome. J Appl Physiol (1985) 116, 439–450 (2014). - PubMed
-
- Zaleski K. L., Kussman B. D., Near-infrared spectroscopy in pediatric congenital heart disease. J. Cardiothorac. Vasc. Anesth. 34, 489–500 (2020). - PubMed
-
- Serena S. B. S., et al. , Young age as a risk factor for impaired cerebral autoregulation after moderate to severe pediatric traumatic brain injury. Anesthesiology J. Am. Soc. Anesthesiologists 108, 588–595 (2008). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

