Cellular proteostasis decline in human senescence
- PMID: 33257563
- PMCID: PMC7749315
- DOI: 10.1073/pnas.2018138117
Cellular proteostasis decline in human senescence
Abstract
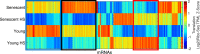
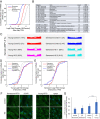
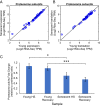
Proteostasis collapse, the diminished ability to maintain protein homeostasis, has been established as a hallmark of nematode aging. However, whether proteostasis collapse occurs in humans has remained unclear. Here, we demonstrate that proteostasis decline is intrinsic to human senescence. Using transcriptome-wide characterization of gene expression, splicing, and translation, we found a significant deterioration in the transcriptional activation of the heat shock response in stressed senescent cells. Furthermore, phosphorylated HSF1 nuclear localization and distribution were impaired in senescence. Interestingly, alternative splicing regulation was also dampened. Surprisingly, we found a decoupling between different unfolded protein response (UPR) branches in stressed senescent cells. While young cells initiated UPR-related translational and transcriptional regulatory responses, senescent cells showed enhanced translational regulation and endoplasmic reticulum (ER) stress sensing; however, they were unable to trigger UPR-related transcriptional responses. This was accompanied by diminished ATF6 nuclear localization in stressed senescent cells. Finally, we found that proteasome function was impaired following heat stress in senescent cells, and did not recover upon return to normal temperature. Together, our data unraveled a deterioration in the ability to mount dynamic stress transcriptional programs upon human senescence with broad implications on proteostasis control and connected proteostasis decline to human aging.
Keywords: UPR; chaperones; heat shock response; protein homeostasis; senescence.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures





Comment in
-
Cellular proteostasis decline in human senescence.Commun Biol. 2021 Jan 4;4(1):17. doi: 10.1038/s42003-020-01578-w. Commun Biol. 2021. PMID: 33398007 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

