Targeting presynaptic H3 heteroreceptor in nucleus accumbens to improve anxiety and obsessive-compulsive-like behaviors
- PMID: 33257584
- PMCID: PMC7749329
- DOI: 10.1073/pnas.2008456117
Targeting presynaptic H3 heteroreceptor in nucleus accumbens to improve anxiety and obsessive-compulsive-like behaviors
Abstract
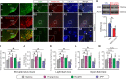
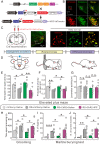
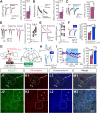
Anxiety commonly co-occurs with obsessive-compulsive disorder (OCD). Both of them are closely related to stress. However, the shared neurobiological substrates and therapeutic targets remain unclear. Here we report an amelioration of both anxiety and OCD via the histamine presynaptic H3 heteroreceptor on glutamatergic afferent terminals from the prelimbic prefrontal cortex (PrL) to the nucleus accumbens (NAc) core, a vital node in the limbic loop. The NAc core receives direct hypothalamic histaminergic projections, and optogenetic activation of hypothalamic NAc core histaminergic afferents selectively suppresses glutamatergic rather than GABAergic synaptic transmission in the NAc core via the H3 receptor and thus produces an anxiolytic effect and improves anxiety- and obsessive-compulsive-like behaviors induced by restraint stress. Although the H3 receptor is expressed in glutamatergic afferent terminals from the PrL, basolateral amygdala (BLA), and ventral hippocampus (vHipp), rather than the thalamus, only the PrL- and not BLA- and vHipp-NAc core glutamatergic pathways among the glutamatergic afferent inputs to the NAc core is responsible for co-occurrence of anxiety- and obsessive-compulsive-like behaviors. Furthermore, activation of the H3 receptor ameliorates anxiety and obsessive-compulsive-like behaviors induced by optogenetic excitation of the PrL-NAc glutamatergic afferents. These results demonstrate a common mechanism regulating anxiety- and obsessive-compulsive-like behaviors and provide insight into the clinical treatment strategy for OCD with comorbid anxiety by targeting the histamine H3 receptor in the NAc core.
Keywords: OCD; anxiety; histamine H3 receptor; nucleus accumbens; prelimbic prefrontal cortex.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Grant J. E., Clinical practice: Obsessive-compulsive disorder. N. Engl. J. Med. 371, 646–653 (2014). - PubMed
-
- de Vries F. E., et al. , Compensatory frontoparietal activity during working memory: An endophenotype of obsessive-compulsive disorder. Biol. Psychiatry 76, 878–887 (2014). - PubMed
-
- Gunaydin L. A., Kreitzer A. C., Cortico-basal ganglia circuit function in psychiatric disease. Annu. Rev. Physiol. 78, 327–350 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

