Neoadjuvant FLOT versus SOX phase II randomized clinical trial for patients with locally advanced gastric cancer
- PMID: 33257672
- PMCID: PMC7705676
- DOI: 10.1038/s41467-020-19965-6
Neoadjuvant FLOT versus SOX phase II randomized clinical trial for patients with locally advanced gastric cancer
Abstract
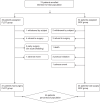
Neoadjuvant chemotherapy with docetaxel, oxaliplatin, fluorouracil, and leucovorin (FLOT regimen) has shown promising results in terms of pathological response and survival rate in patients with locally advanced resectable gastric cancer (LAGC). However, tegafur gimeracil oteracil potassium capsule (S-1) plus oxaliplatin (SOX regimen) is the preferred chemotherapy regimen in Eastern countries. Here, we conduct an open label, two-arm, phase II randomized interventional clinical trial (Dragon III; ClinicalTrials.gov: NCT03636893) to evaluate the safety and efficacy of both regimens. Patients with LAGC are randomly assigned to receive either 4 cycles of the neoadjuvant FLOT regimen (40 patients) or 3 cycles of the SOX regimen (34 patients) before gastrectomy. The primary endpoint is the comparison of complete (TRG1a) or subtotal (TRG1b) tumor regression grading in the primary tumor. There are no significant differences in adverse effects or postoperative morbidity and mortality between the two groups. No significant differences in the proportion of tumor regression grading between the FLOT group and the SOX group are found. Complete or subtotal TRG is 20.0% in the FLOT group versus 32.4% in the SOX group. Therefore, our study does not find statistically significant differences between neoadjuvant FLOT and SOX regimens for the primary outcomes reported here in locally advanced gastric cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous