New solar energy-storage resource of plasmon-activated water solution with higher chemical potential
- PMID: 33257784
- PMCID: PMC7705734
- DOI: 10.1038/s41598-020-77815-3
New solar energy-storage resource of plasmon-activated water solution with higher chemical potential
Abstract
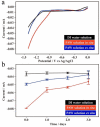
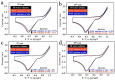
Nowadays, solar energy is the most environmentally friendly energy source to drive many chemical reactions and physical processes. However, the corresponding fabrication procedures for obtaining excellent energy-storage devices are relatively complicated and expensive. In this work, we report an innovative strategy on plasmon-activated water (PAW) serving as energy-storage medium from solar energy. The lifetime of the created energetic PAW solution from hot electron transfer (HET) on Au nanoparticles (AuNPs) illuminated with sunshine can last for 2 days, making the energy-storage system is practically available. Encouragingly, the energy-conversion efficiency from the solar energy in the PAW solution is ca. 6.7%. Compared to conventional deionized (DI) water solution, the prepared metastable PAW solution exhibited distinctly higher chemical potential at room temperature. It demonstrates abilities in faster evaporation and enhancing chemical reactions, including hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). Our proposed strategy on the simple and cheap energy-storage system based on prepared PAW utilizing solar energy is the first time shown in the literature.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
LinkOut - more resources
Full Text Sources

